|
Properties
|
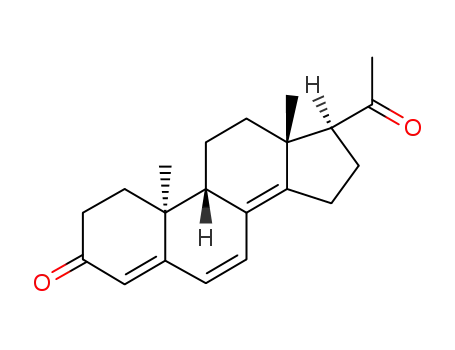
Dydrogesterone is a stereoisomer of progesterone with an additional double bond between C6 and C7, and its hormonal pattern and metabolism differ largely from those of the natural progestogen. It is an orally active progestin that is non-thermogenetic, non-sedative and does not inhibit gonadotropin release and ovulation. It has weak antimineralocorticoid effects, negligible androgenic and glucocorticoid activities, and no antiandrogenic properties[4]. Oral treatment with 10–20 mg dydrogesterone daily caused a sufficient secretory transformation of a proliferated endometrium. The half-life[t1/2b] is 5–7 h and 24 h after oral administration, and within 24 h 85% of the dose is excreted. Due to the 9b, 10a-retro structure of the molecule, both double bonds cannot be enzymatically reduced. The most important metabolic steps are the reduction of the 20-keto groups, and hydroxylation at C16a and C21. The main metabolite is 20a-dihydrodydrogesterone.
The sequential therapy of postmenopausal women with either 1 mg estradiol and 5 or 10 mg dydrogesterone or 2 mg estradiol and 10 or 20 mg dydrogesterone caused an atrophic or secretory endometrium in most patients and prevented the development of endometrial hyperplasia[5].
The continuous combined oral treatment of postmenopausal women with 1 mg estradiol and 2.5 mg, 5 mg, 10 mg, or 20 mg dydrogesterone daily caused an increase in HDL cholesterol and a decrease in LDL cholesterol. The results suggest that dydrogesterone enhanced the estrogeninduced decrease in LDL cholesterol and attenuated that on HDL cholesterol[6]. |
|
Indications
|
Dydrogesterone is used for the treatment of a variety of indications, including threatened or recurrent miscarriage during pregnancy, dysfunctional bleeding, infertility due to luteal insufficiency, dysmenorrhea, endometriosis, secondary amenorrhea, irregular cycles, premenstrual syndrome, and as a component of menopausal hormone therapy[7]. |
|
Pharmacological Aspects
|
Dydrogesterone is an orally active progestogen that acts directly on the uterus, which can produce a complete secretory endometrium in an estrogen-primed uterus. Dydrogesterone has no contraceptive effect as it does not inhibit or interfere with ovulation or the corpus luteum. Furthermore, dydrogesterone is non-androgenic, non-estrogenic, non-corticoid, non-anabolic and is not excreted as pregnanediol. Dydrogesterone can assist in regulating the healthy growth and normal shedding of the uterus lining. Therefore, it may be useful in the treatment of menstrual disorders such as absent, irregular or painful menstrual periods, infertility, premenstrual syndrome and endometriosis[3].
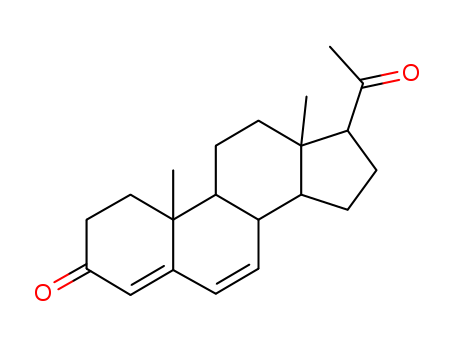
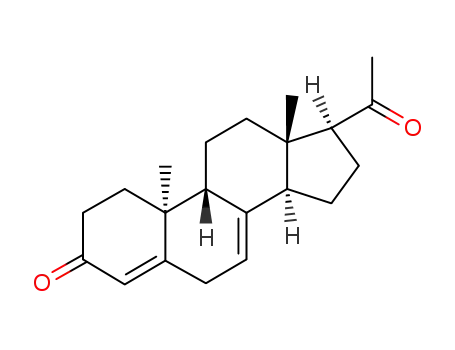
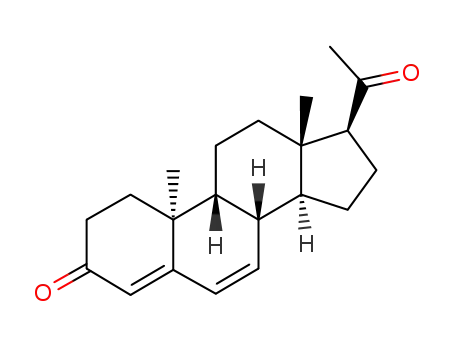
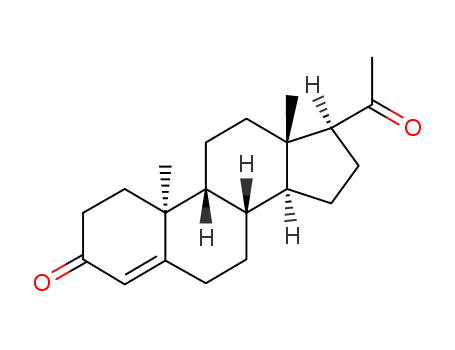
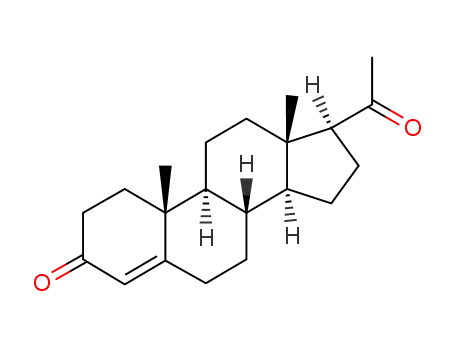
Dydrogesterone is an orally active 6-dehydro retroisomer of natural progesterone[fig. 1]. Dydrogesterone has progestational and antiestrogenic activity, but no significant estrogenic activity. Dydrogesterone is not chemically related to testosterone and its low affinity for the androgen receptor means that it has neither significant androgenic nor antiandrogenic activity[8]. In contrast, most 19-nortestosterone derivatives have androgenic activity[9] and thus tend to negate some of the beneficial effects of estrogen on lipid and lipoprotein levels[10] and to adversely affect glucose metabolism[11]. This does not occur with dydrogesterone.
Dydrogesterone does not have significant glucocorticoid, antimineralocorticoid, and anti-inflammatory or thymolytic effects[12]. |
|
Pharmacodynamic profile
|
Absorption and distribution
Orally active, in contrast to natural progesterone[22]
Tmax of dydrogesterone and its major metabolite DHD ~0.5 to 2.5 hours[23]
Half-life of dydrogesterone ranges ~5 to 7 hours[23]
Metabolism
43 metabolites identified[24]
Most important metabolite is DHD[24]
AUC for DHD is approximately 30to 40-fold greater than that of dydrogesterone after PO administration of dydrogesterone[24]
Half-life~ of DHD ~14 to 17 hours[23]
No aromatised metabolites, which accounts for the lack of estrogenic activity[24]
No 17a-hydroxylation, which accounts for the lack of androgenic activity[24]
Excretion
Mainly excreted as metabolites in the urine[~63% of dose][24]
Urinary excretion almost complete within 72 hours[24] |
|
Mechanisms of action
|
Dydrogesterone is a kind of progestogens derivatives, sharing similar mechanism of action. Originally, progestogens were defined as compounds which maintain pregnancy, but, in the human, only progesterone can maintain pregnancy. The activity and potency of synthetic progestins are mostly evaluated by means of parameters associated with endometrial effects.
The various actions of progesterone and synthetic progestins are brought about by genomic interactions with the progesterone receptors[PRs], which exist in the two isoforms, PRA and PRB, and by rapid non-genomic interactions with membrane binding sites. Moreover, according to their chemical structure, progestogens may bind to other members of the nuclear receptor superfamily. Binding of a progestogen to the receptor results in dimerization and interaction with a hormone responsive element within hormoneregulated target genes. In general, PRA may act as transcriptional repressor and PRB as activator. PRA may repress not only the transcriptional activity of the PRB, but also that of the ER, androgen receptor, and the glucocorticoid and mineralocorticoid receptors[25].
In most tissues, the biological action of progestogens is dependent on the presence of estrogens, as estrogens play a key role in the induction of PR, while progestogens down-regulate the expression of the ER. Progestogens may also reduce the expression of PR in the endometrial epithelium, but not the stromal and myometrial PR. Both PRA and PRB are expressed in the endometrial glands, whereas PRA predominates in the endometrial stroma[26]. In the breast of primates, progestogens may reduce the expression of the ERa and PR, but the estrogen-induced proliferation of the mammary epithelium is not inhibited, but enhanced by progestogens[27].
The primary role of progestogens in HRT is the inhibition of estrogen-induced proliferation of the endometrium. Moreover, they induce secretory changes in a proliferated endometrium. The antiestrogenic effect of progestogens in the endometrium is associated with a suppression of ER and the activation of the 17b-HSD type 2 which converts estradiol to estrone, and the estronesulfotransferase which causes conjugation of estrone. |
|
Adverse reactions
|
Potential side effects of dydrogesterone may include pulsating headache which can develop into a migraine, pain in breast and breast tenderness, occasional blood spotting, pain during periods, irregular period, heavy bleeding during periods, missed period, weight gain, depression, abnormal liver function which can progress into, jaundice nausea and abdominal discomfort as well as hypersensitivity[28]. |
|
Precaution
|
Following tips should be followed[28]:
Patients should be monitored for visual disturbances, migraine headache or embolic disorders. The duration and the frequency of the treatment should be decided by the treating physician. If any allergic reaction reactions occur, the medicine should be stopped immediately and should consult a physician. Caution should be exercised in patients with a history of blood clot disorder, kidney impairment, heart disease, epilepsy, asthma, migraine, fluid retention, and breastfeeding. Monitor closely eye vision and symptoms of blood clot disorders. It may cause dizziness, do not drive a car or operate machinery while taking this medication. It should be used with extreme care in patients with a history of clinical depression. |
|
Reference
|
E. Reerink, H. Schoeler, P. Westerhof, A. Querido, A. Kassenaar, E. Diczfalusy, et al., A new class of hormonally active steroids, Nature 186[1960] 168–169.
G.J. Macdonald, Maintenance of pregnancy in ovarectomized rats with steroid analogs and the reproductive ability of the progeny, Biol. Reprod. 27[1982] 261–267.
https://www.drugbank.ca/drugs/DB00378
Tausk M, de Visser J. Pharmacology of orally active progestational compounds: animal studies. In Tausk M, ed. Pharmacology of the Endocrine System and Related Drugs: Progesterone, Progestational Drugs and Antifertility Agents. Oxford: Pergamon Press, II: 35–216
Ferenczy A, Gelfand MM, van de Weijer PHM, et al. Endometrial safety and bleeding patterns during a 2-year study of 1 or 2 mg 17bestradiol combined with sequential 5–20 mg dydrogesterone. 6. Climacteric 2002;5:26–35?
Pornel B, Chevallier O, Netelenbos JC. Oral 17b-estradiol[1 mg] continuously combined with dydrogesterone improves the serum lipid profile of postmenopausal women. Menopause 2002;9:171–8
Kuhl H[2005]. "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 Suppl 1: 3–63.
Vermorken AJM, Sultan CH, Goos CMAA. Dydrogesterone has no peripheral[anti]-androgenic properties. In Vivo 1987; I: 167-72
Rozenbaum H. Relationships between chemical structure and biological properties of progestogens. Am J Obstet Gynecol 1982 Mar 15; 142[6 Pt 2]: 719-24
Rijpkema AHM, van der Sanden AA, Ruijs AHC. Effects of post-menopausal oestrogen-progestogen replacement therapy on serum lipids and lipoproteins: a review. Maturitas 1990; 12[3]: 259-85
Baird DT, Fraser IS. Blood production and ovarian secretion rates of estradiol-17~ and estrone in women throughout the menstrual cycle. J Clin Endocrinol Metab 1974; 38[6]: 100917
Eckerniiss-A,GrahnenA. A study on multiple dose kinetics of dydrogesterone and dihydrodydrogesterone at 20 mg dydrogesterone and pharmacokinetic interactions between dydrogesterone[20 mg] and estradiol[2 mg] at steady state. Solvay Duphar B.V.[Weesp, The Netherlands]. 102.6004; 1990[Data on file]
Scholer HFL. Biological properties of 9,IO-isomeric steroids. I. Progestational activity of9beta,I0 alpha-steroids. Acta Endocrinol 1960; 35: 188-96
Lane G, Siddle NC, Ryder TA, et al. Effects of dydrogesterone on the oestrogenized postmenopausal endometrium. Br J Obstet Gynaecol 1986; 93[I]: 55-62
Suchowsky GK, Turolla E, Arcari G. Studies of the so-called virilizing effects of steroids in female rat fetuses. Endocrinology 1967 Feb; 80: 255-62
Boris A, Stevenson RH, Trmal T. Some studies of the endocrine properties of dydrogesterone. Steroids 1966 Jan; 7: 1-10
Marois M. Absence de proprietes masculinisantes ou feminisantes d'un progestatif actif per os, la dydrogesterone chez Ie rat mate ou femelle adulte et chez Ie foetus de rat. Bull Acad Natl Med 1962; 146: 354-64
SchOler HFL, de Wachter AM. Evaluation of androgenic properties of progestational compounds in the rat by the female foetal masculinization test. Acta Endocrinol 1961; 38: 128-36
Jost A. Maintien de la gestation chez la lapine par un stereoisomere voisin de la progesterone[6-dehydro-retroprogesterone]. Action sur les foetus. Acta Endocrino1 1963; 43: 539-44
Verhaar HJJ, Damen CA, Duursma SA, et al. A comparison of the action of progestins and estrogen on the growth and differentiation of normal adult human osteoblast-like cells in vitro. Bone 1994; 15[3]: 307-11
Roux C, Kolta S, Chappard C, et al. Bone effects of dydrogesterone in ovariectomized rats: a biologic, histomorphometric, and densitometric study. Bone 1996 Nov; 19: 463-8
Tillinger K-G, Diczfalusy E. Progestational activity of stereoisomeric progesterone-analogues following oral administration in amenorrhoea. Acta Endocrino] 1960; 35: 197-203
Femoston?. 17~-estradiol + dydrogesterone: product monograph. Weesp, The Netherlands: Solvay Duphar B.V., 1996
24. van Amsterdam PH, Overmars H, Scherpenisse PM, et al. Dydrogesterone: metabolism in man. Eur J Drug Metab Pharmacokinet 1980; 5[3]: 173-84
Giangrande PH, McDonnell DP. The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Progr Horm Res 1999;54:291–313
Kuhl H. Pharmacology of progestogens. Basic aspects – progesterone derivatives. Menopause Rev 2001;6:9–16
Cline JM, Soderqvist G, von Schoultz E, et al. Effects of hormone replacement therapy on the mammary gland of surgically postmenopausal cynomolgus macaques. Am J Obstet Gynecol 1996;174:93–100
https://www.medindia.net/doctors/drug_information/dydrogesterone.htm#Sideeffects |
|
Manufacturing Process
|
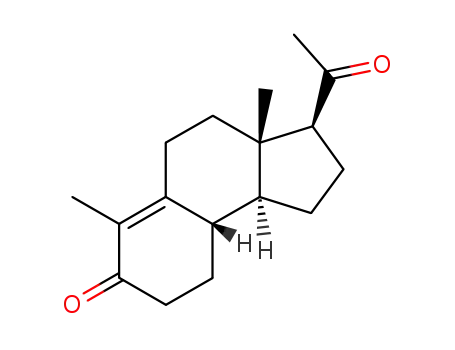
A solution of 7.5 grams of retroprogesterone in 500 ml of freshly distilled
tertiary butyl alcohol was refluxed with 12.75 grams of finely powdered
chloranil, while stirring, for 5 hours in a nitrogen atmosphere. After cooling, 2
liters of water were added and extraction was performed three times with 200
ml of methylene dichloride. The combined extracts were then diluted with 1
liter of petroleum ether (40-60°C) washed successively with 100 ml of diluted
Na2SO4, four times with 75 ml of 1 N NaOH, and then water to neutral
reaction.By drying this solution on Na2SO4, and evaporating to dryness (last part in
vacuo) 3.7 grams of crystalline residue was obtained. This residue was then
dissolved in benzene. Filtration in benzene filtered through 35 grams of
alumina (according to Brockmann was done and then the alumina was eluted
with benzene. Evaporation of the benzene yielded 3.11 grams of crystalline
residue. By crystallization with 15 ml of acetone at room temperature (at
lower temperatures a by-product crystallized out) 900 mg of crystals, with a
melting point of 165°-170°C were obtained. Transfer of the acetone mother
liquor into a mixture of ethanol and hexane yielded 1.7 grams of a solid
substance with a melting point of 130° to 145°C. This solid was then
recrystallized with acetone at room temperature, yielding 600 mg of a solid
with a melting point of 166° to 171°C. The two fairly pure fractions (600 mg
and 900 mg) yielded, after crystallization with a mixture of acetone and
hexane, finally 1.0 gram of 6-dehydroretroprogesterone, melting point 169° to
170°C. From the mother liquors an additional fraction of 0.44 gram with a
melting point of 168° to 169°C was obtained. |
|
Therapeutic Function
|
Progestin |
|
Overview
|
Dydrogesterone is a synthetic progestational hormone with no androgenic or estrogenic properties. Unlike many other progestational compounds, dydrogesterone produces no increase in temperature and does not inhibit ovulation.
Dydrogesterone belongs to the “retrosteroid” class and is a stereoisomer of natural progesterone. Its conformation is induced by exposing progesterone to ultraviolet light, a reaction first described in the 1950s by Reerink et al. who also discovered this new class of functionally active “retrosteroids”[1]. Dydrogesterone shares similar biological properties with natural progesterone and has high affinity for progesterone receptors [PRs]. In contrast to progesterone, dydrogesterone is highly bioavailable after oral intake and is therefore available on the market as an orally active progestogens [Duphaston?]. Progesterone was recognised early on to be one of the most important steroids required for the maintenance of pregnancy, and so dydrogesterone became a candidate for the treatment of threatened and habitual abortion. As early as the beginning of the 1980s, the pregnancymaintaining effects of dydrogesterone were demonstrated in ovariectomised rats, although the underlying mechanism was unknown[2]. In contrast to other progestogens on the market, dydrogesterone has no relevant androgenic or antiandrogenic activity on the foetus and can therefore be safely administered to mothers without the risk of causing foetal genital malformations.
It is used for the treatment of irregular duration of cycles and irregular occurrence and duration of periods caused by progesterone deficiency. It is also used to prevent natural abortion in patients who have a history of habitual abortions [3].
Figure 1 the chemical structure of dydrogesterone ; |
|
Brand name
|
Gynorest (Solvay Pharmaceuticals). |