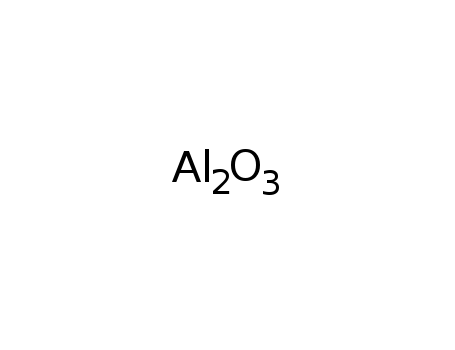
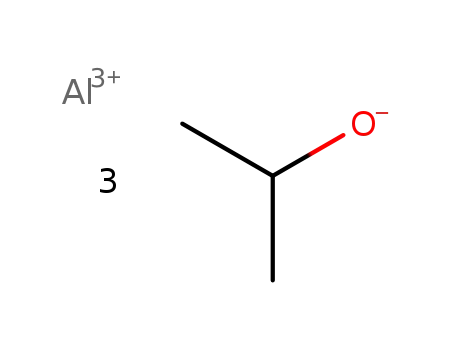
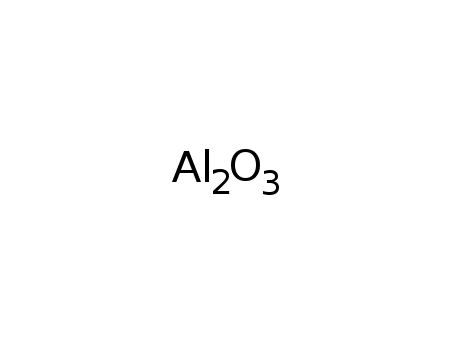
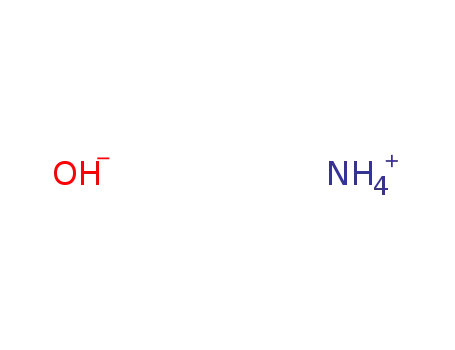
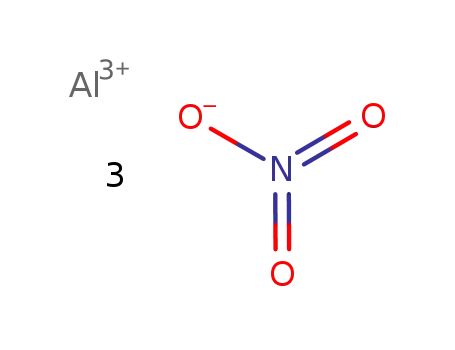
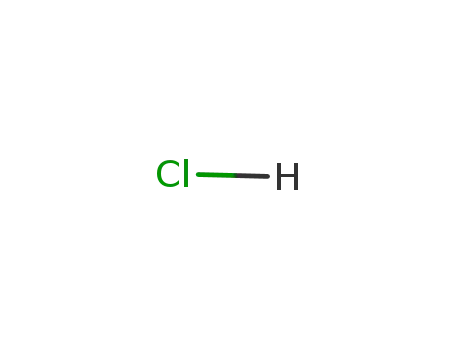
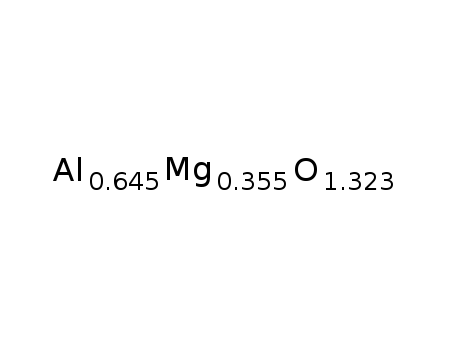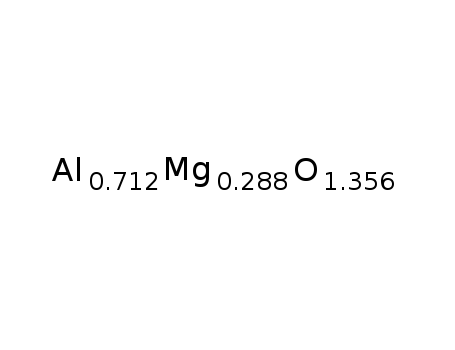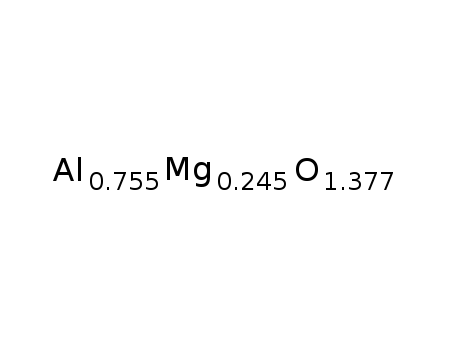| Conditions |
Yield |
|
In
water; isopropyl alcohol; toluene;
at 265 ℃;
for 14h;
under 45004.5 - 75007.5 Torr;
|
|
|
hydrolysis;;
|
|
|
reduced-pressure chemical vapor deposition (N2, N2/H2, N2/O2 or N2/H2O atmoisphere, 200-380°C, total gas flow 1000 ml/min);
|
|
|
With
water;
In
propan-1-ol;
pptn. on H2O addn., polymer addn. either with H2O or after hydrolysis step, drying, calcination (773 K, 24 h); surface area and porosity depending on polymer type, its amount, and method of addition;
|
|
|
With
polyethylene glycol; water;
In
propan-1-ol;
pptn. on H2O addn., polymer addn. either with H2O or after hydrolysis step, drying, calcination (773 K, 24 h); surface area and porosity depending on polymer type, its amount, and method of addition;
|
|
|
With
water;
In
propan-1-ol;
pptn. on H2O addn., polymer addn. either with H2O or after hydrolysis step, drying, calcination (773 K, 24 h); surface area and porosity depending on polymer type, its amount, and method of addition;
|
|
|
With
water; Polyvinyl alcohol;
In
propan-1-ol;
pptn. on H2O addn., polymer addn. either with H2O or after hydrolysis step, drying, calcination (773 K, 24 h); surface area and porosity depending on polymer type, its amount, and method of addition;
|
|
|
With
water;
In
ethanol; water;
hydrolyzing Al-compd. dissolved in dried EtOH with distilled water; evapn., suspending of residue in distilled water and boiling for 1 h, evapn.;
|
|
|
With
water;
In
neat (no solvent);
slow hydrolysis of aluminium isopropoxide (placed in a porcelein dish) by atmospheric moisture, solid transferred to a silica crucible, dried in an air oven, 100 ° C, 12 h, calcined in a furnace, 600 ° C, 6 h;
|
|
|
With
water;
In
propan-1-ol;
pptn. on H2O addn., drying, calcination (773 K, 24 h); surface area and porosity depending on polymer type, its amount, and method of addition;
|
|
|
With
water;
In
not given;
hydrolysis, calcination of the obtained ppt. (800°C, 6 h); predominant γ-Al2O3;
|
|
|
With
oxygen;
In
gaseous matrix;
chemical vapor deposition (polycrystalline Pt or single-crystal Si substrate, 623 K, N2 carrier gas);
|
|
|
With
air;
In
cyclohexane;
dissoln. of Al compd. in C6H12, room temp.; stirring, 20-30 min; spraying through nozzle; freezing in stirred liq. nitrogen; decanting; drying by sublimation; calcining in flowing air at 300-800.degree;
|
|
|
With
air;
In
not given;
Al(OC3H7)3 hydrolysed, heated at 600°C for 24 h, pressed into disc, crushed, sieved, heated at 800°C for 4 h in air;
|
|
|
In
gas;
byproducts: AlO(OH), propene, H2O; thermal decompn. at 300-360°C, further products propane, isopropanol, acetone; elem. anal.;
Kinetics;
|
|
|
In
gaseous matrix;
chemical vapor deposition (N2 carrier gas, total flow rate 1000 ml/min,silicon substrate, 250-450°C=;
|
|
|
In
ethanol;
soln. of metal compds. sprayed onto substrate keeping over 400°C by Kavan, L. Graetzel, M., Electrochim. Acta 1995, 40, 643; annealed at 500°C for 1 h, analyzed by XRD; metal oxide layer obtained;
|
|
|
In
water;
hydrolysis, calcination (550°C) according to Lahousse, C., Aboulayt, A., Mauge, F., Bachelier, J. and Lavalley, J. C., J. Mol. Catal. 74,283 (1993).;
|
|
|
In
water;
hydrolyzation (85°C, 30 min) with water, drying (room temp., 24 h, 110°C, 12 h), calcination (500°C, 5 h) in air flow;
|
|
|
In
ethanol; water;
hydrolysis, pptn. of aluminium hydroxide, drying (130°C, 6 h);
|
|
|
In
neat (no solvent);
Electric Arc; low-temp. plasma deposition, pyrolysis, 290 and 460°C, atmospheric pressure, annealing (510°C, 15 min);
|
|
|
In
neat (no solvent);
thermal decompn. at 360°C, calcination at 600°C;
|
|
|
In
water; isopropyl alcohol;
calcination (600°C, 16 h;
|
|
|
In
propan-1-ol;
dissolving under stirring, water slow addn., slow agitation (24 h), filtration, washing (water), drying (373 K, 24 h), calcination (773 K, 24 h);
|
|
|
In
octane;
CVD on Si wafer at 300-475°C; soln. of Al isopropoxide in n-octane vaporized at 210°C; carrier gas: Ar; deposition pressure: 1.2 Torr; annealed in O2 for 1 h;
Kinetics;
|
|
|
With
Pluronic P123 triblock copolymer;
In
not given;
addn. of Pluronic P123 to a soln. of aluminum isopropoxide with or without polystyrene beads, slow evapn., calcination;
|
|
|
hydrolyzing; washing, drying;
|
|
|
In
water;
purified liquid poured into deionized water, stirred, form gel of aluminum hydroxide; hydroxide decomposed in air at 850°C;
|
|
|
With
water;
In
water;
hydrolysis; washing with water and drying at 120°C;
|
|
|
With
H2O;
In
water;
hydrolysis react., pptd. Al(OH)3 filtered, washed thoroughly, cut into cakes, dried at 120°C for 24 h, activated at 500°C in a stream of pure air for another 24 h; crushed, powdered and sieved to 40-60 mesh size;
|
|
|
hydrogenchloride;
In
water;
stirred under N2 at 170-250 °C, evapd., calcined at 1200 °C for 2 h;
|
|
|
With
H2O;
In
water;
Al isopropoxide addn. to water, heating (90°C, 2 h, vigorous stirring), HCl addn., boiling, dip coating (Si3N4 substrate), firing (1500°C, 10 h); scanning electron microscopy;
|
|
|
In
isopropyl alcohol;
(N2), Al isopropoxide refluxing in isopropanol( (overnight), H2O/isopropanol dropwise addn. (vigorous stirring), refluxing (1 h), evapn. (30°C), drying (105°C, overnight), calcination (400, 600, 800, or1000°C, 6 h, air); X-ray diffraction;
|
|
|
With
lauric acid; water;
In
propan-1-ol;
Al-hydroxide suspn. prepd. by hydrolysis of Al-isopropoxide with water in propanol; suspn. stirred for 1 h and then lauric acid added slowly; mixt. aged for 24 h and heated at 110°C for 2 d; ppt.filtered, washed with ethanol and dried; compd. calcined at temp. of 450°C with temp. ramp of 1°C/min under N2 (<200°C) and air at (>200°C);
|
|
|
In
further solvent(s);
High Pressure; according to Iwamoto, S.; Inoue, M. J. Jpn. Pet. Inst. 2008, 51, 143; aluminum triisopropoxide suspended in diethylenetriamine; placed in autoclave; diethylenetriamine added; purged with N2; heated (300°C, 2 h); cooled to room temp.; washed with acetone; air-dried; calcined (700°C, 30 min);
|
|
|
aluminum isopropoxide;
With
nitric acid;
In
ethanol;
for 5h;
In
ethanol;
at 60 ℃;
for 96h;
|
|
|
aluminum isopropoxide;
With
nitric acid;
In
ethanol;
for 5h;
at 60 ℃;
for 48h;
at 900 ℃;
for 10h;
Calcination;
|
|
|
aluminum isopropoxide;
With
cetyltrimethylammonim bromide;
In
water; isopropyl alcohol; toluene;
at 20 ℃;
for 29.5h;
at 500 ℃;
for 5h;
Calcination;
|
|
|
aluminum isopropoxide;
With
nitric acid;
In
ethanol;
at 20 ℃;
for 5h;
at 60 ℃;
for 48h;
Calcination;
|
|
|
aluminum isopropoxide;
With
nitric acid;
In
ethanol;
at 20 ℃;
for 5h;
at 400 ℃;
for 4h;
Calcination;
|
|
|
With
Pluronic P104;
In
ethanol;
at 20 ℃;
for 5h;
|
|
|
aluminum isopropoxide;
With
ammonium hydroxide; cis-Octadecenoic acid;
at 79.84 - 179.84 ℃;
for 53h;
at 549.84 ℃;
Calcination;
|
|
|
aluminum isopropoxide;
With
nitric acid;
In
ethanol;
at 60 ℃;
for 48h;
at 20 - 800 ℃;
Calcination;
|
|
|
aluminum isopropoxide;
With
nitric acid;
In
ethanol;
for 12h;
at 400 ℃;
for 2h;
Calcination;
|
|
|
aluminum isopropoxide;
With
nitric acid;
In
ethanol;
at 20 ℃;
for 5h;
at 700 ℃;
for 4h;
Calcination;
|
|
|
aluminum isopropoxide;
With
nitric acid;
In
ethanol;
at 60 ℃;
for 48h;
at 700 ℃;
for 4h;
Calcination;
|
|
|
With
water;
at 550 ℃;
for 2h;
|
|