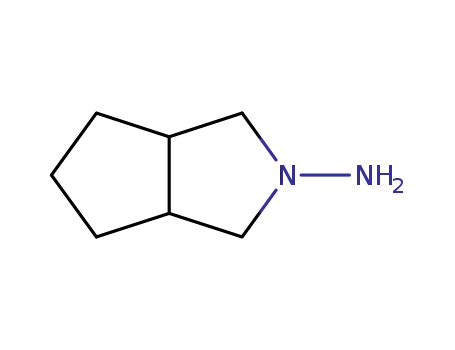
Reputable manufacturer supply 3-Amino-3-azabicyclo[3.3.0]octane hydrochloride 58108-05-7 in stock with high standard
- Molecular Formula:C7H14N2.HCl
- Molecular Weight:162.662
- Appearance/Colour:light yellow brown powder
- Melting Point:170-174 °C(lit.)
- Boiling Point:229.9 °C at 760 mmHg
- Flash Point:92.9 °C
- PSA:29.26000
- LogP:2.03220
3-Amino-3-azabicyclo[3.3.0]octane hydrochloride(Cas 58108-05-7) Usage
|
General Description
|
3-Amino-3-azabicyclo[3.3.0]octane hydrochloride is also referred to as N-amino-3-azabicyclo[3.3.0]octane monohydrochloride. Jilin Tianyun New Materials Co., Ltd. is a professional production enterprise that production, processing, and export, mainly engaged in various organic chemicals. We look forward to establishing a long-term stable and win-win business relationship with you.
|
| Uses |
3-Amino-3-azabicyclo[3.3.0]octane hydrochloride is used as a reactant in the synthesis of cannabinoid receptor antagonists. 3-Amino-3-azabicyclo[3.3.0]octane hydrochloride is used as an intermediate in the synthesis of Gliclazide, which is used to treat type 2 diabetes. |
InChI:InChI=1/C7H14N2.ClH/c8-9-4-6-2-1-3-7(6)5-9;/h6-7H,1-5,8H2;1H
58108-05-7 Relevant articles
Synthesis process of N-amino-3-azabicyclo [3, 3, 0] octane hydrochloride
-
Paragraph 0017; 0027; 0030-0032; 0035-0037; 0040-0042; 0045-, (2021/05/29)
The invention provides a synthesis proce...
Method for preparing N-amino-3-aza-bicyclo [3, 3, 0] octane hydrochloride
-
Paragraph 0030-0032, (2018/10/19)
The invention relates to a new method fo...
Applications of biological of Azo-Schiff base ligand and its metal complexes and: A review.
Ashoor, Lamia S.; Majeed, Rawa'a Abass; Al-Shemary, Rehab Kadhim Raheem
, AL-Muthanna Journal of Pure Science, 2021, Vol 8, Issue 1, p74
In 2016 Selma B[25], was prepared a novel azoSchiff base applying salicylaldehyde, aniline and 3-amino-3-azabicyclo[3.3.0]octane hydrochloride. The ligand were reacted with of Ni (II...
Influence of blood proteins on biomedical analysis. IX. Protective effects of human serum proteins on anion-induced degradation of gliclazide
Kobayashi,Igaki,Kimura,Sakoguchi,Shimosawa,Matsuoka
, p. 2957 - 2962 (2007/10/02)
-
58108-05-7 Process route
-
- 1050666-51-7
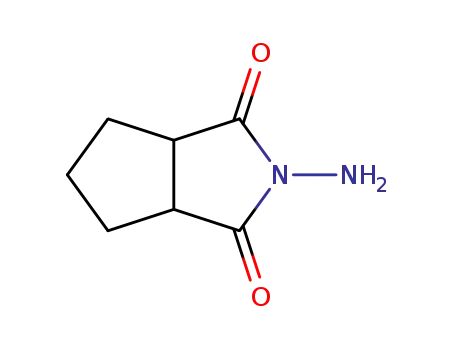
N-amino-1,2-cyclopentanedicarboximide
-
- 58108-05-7
3-azabicyclo<3.3.0>oct-3-yl-amine monohydrochloride
Conditions
| Conditions |
Yield |
|
N-amino-1,2-cyclopentanedicarboximide; With aluminum (III) chloride; In tetrahydrofuran; at 45 ℃; for 0.333333h; Large scale;
With sodium tetrahydroborate; In tetrahydrofuran; for 6h; Reflux; Large scale;
With hydrogenchloride; In tetrahydrofuran; pH=3; Solvent; Temperature; Large scale;
|
81.3% |
|
Multi-step reaction with 2 steps
1: acetic acid; pyrographite; ruthenium(III) chloride trihydrate; hydrogen / water / 16 h / 140 °C / 60006 Torr / Autoclave
2: hydrogenchloride
With hydrogenchloride; ruthenium(III) chloride trihydrate; hydrogen; pyrographite; acetic acid; In water;
|
|
-
-
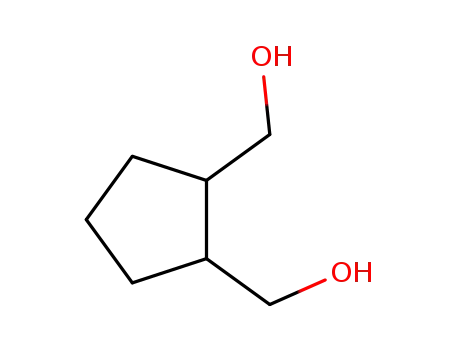
cis-cyclopentane-1,2-dimethanol
-
- 58108-05-7
3-azabicyclo<3.3.0>oct-3-yl-amine monohydrochloride
Conditions
| Conditions |
Yield |
|
With hydrogenchloride; hydrazine hydrochloride; In water; at 140 ℃; for 5h; under 1672.11 - 4256.29 Torr; Autoclave;
|
|
58108-05-7 Upstream products
58108-05-7 Downstream products

![3-Amino-3-azabicyclo[3.3.0]octane hydrochloride](/upload/2024/8/9921ed39-997b-4c5b-8e0e-1dbf93f04e8b.png)