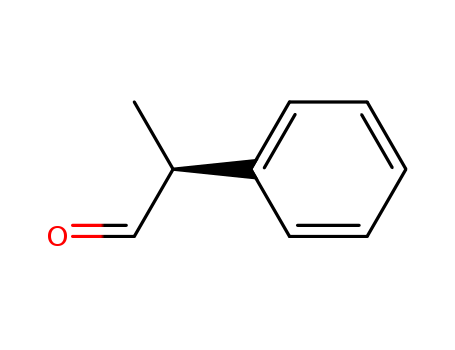
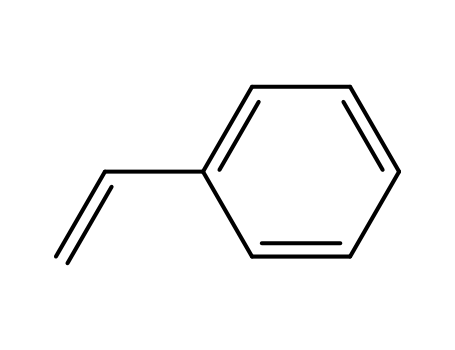
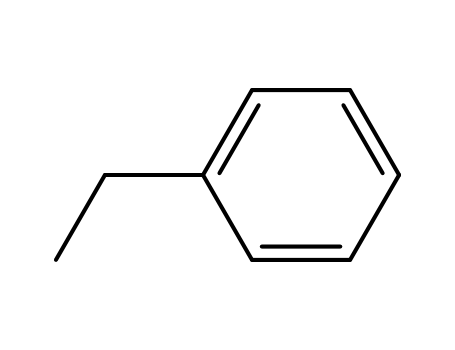
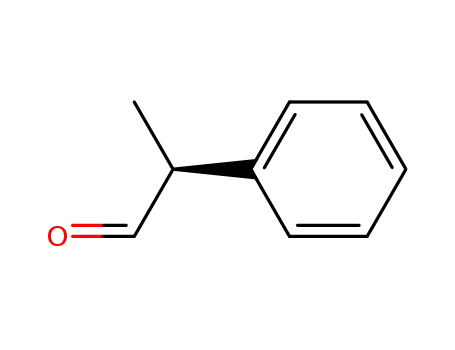
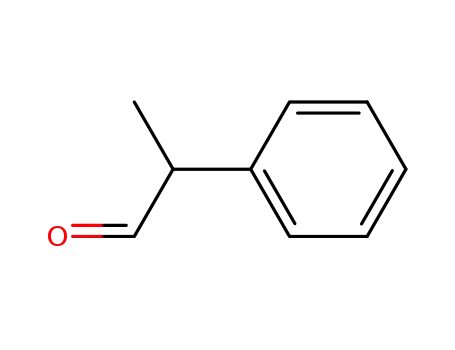
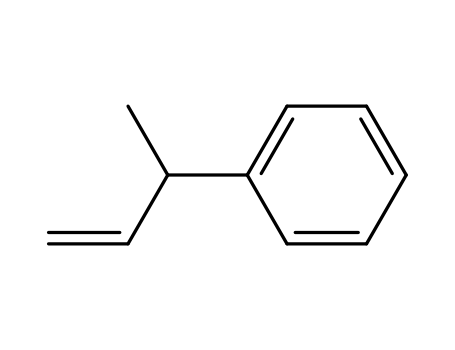
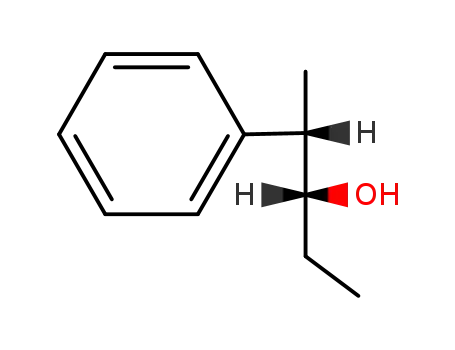
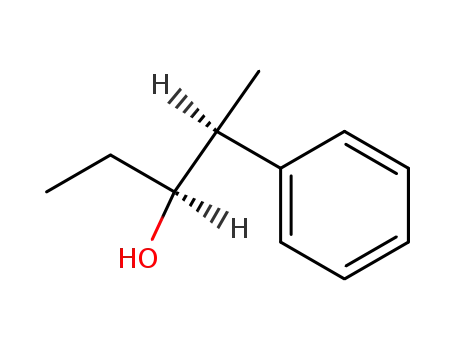
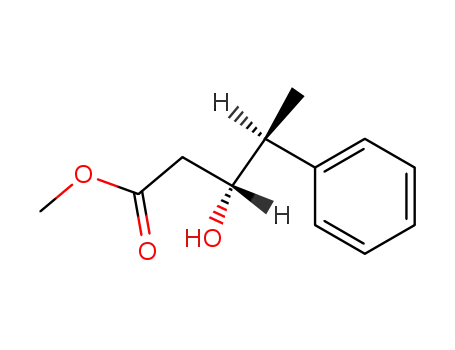
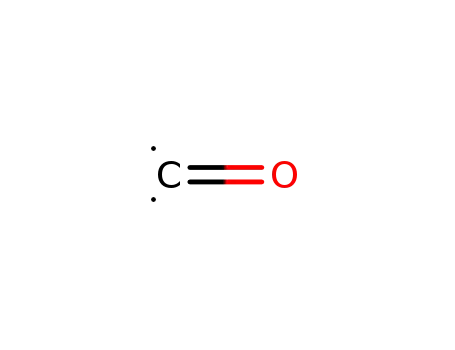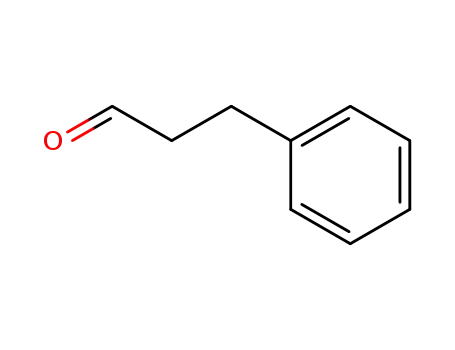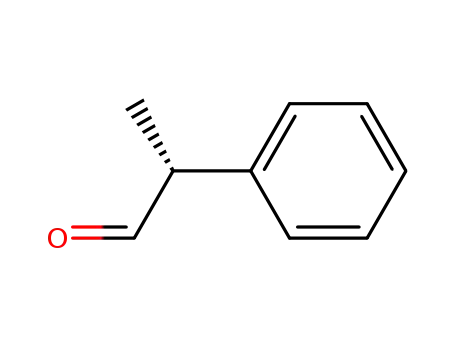| Conditions |
Yield |
|
With [(2S,3S)-2,4-bis(diphenylphosphino)pentane]Pt(SnCl3)Cl; hydrogen; In toluene; at 40 ℃; for 138h; under 51714.8 Torr;
|
68.7 % Chromat.
25%
6.3% |
|
With hydrogen; PtCl2; In toluene; at 40 ℃; for 160h; under 60004.8 Torr; Product distribution; var. PtCl2-diphosphine 2,4-bis(diphenylphosphino)pentane (BDPP)-tin(II)fluoride catalytic system; other temp. and time;
|
|
|
With hydrogen; bis(benzonitrile)dichloroplatinum(II); tin(ll) chloride; (2S,4S)-2,4-bis[((S)-dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-2-yl)oxy]pentane; In toluene; at 100 ℃; for 2h; under 76000 Torr; Title compound not separated from byproducts;
|
|
|
With hydrogen; bis(benzonitrile)dichloroplatinum(II); tin(ll) chloride; (2S,4S)-2,4-bis[((S)-dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-2-yl)oxy]pentane; In toluene; at 17 ℃; for 70h; under 76000 Torr; Title compound not separated from byproducts;
|
|
|
With chiral bis(octahydrodinaphthodioxaphosphepin)-based ligand; hydrogen; tin(ll) chloride; bis(benzonitrile)dichloroplatinum(II); In toluene; at 23 ℃; for 20h; Title compound not separated from byproducts;
|
|
|
With hydrogen; [Rh(NBD)(S)-BINAPO]BF4; In toluene; at 40 ℃; for 15h; under 60004.8 Torr; Further Variations:; Catalysts; Solvents; Temperatures; Product distribution;
|
|
|
With hydrogen; chiral bis(phosphite)PtCl2-SnCl2; In toluene; at 100 ℃; for 2h; under 76000 Torr; Product distribution; other temp., times, solvent and catalyst;
|
|
|
With (2S,4S)-2-(dibenzophospholyl)-4-(diphenylphosphino)pentane; hydrogen; tin(ll) chloride; bis(benzonitrile)dichloroplatinum(II); In toluene; at 24 ℃; for 45h; under 148200 Torr; Further Variations:; Catalysts; Temperatures; Pressures; Kinetics; Product distribution;
|
|
|
With hydrogen; tris(pentafluorophenyl)borate; In toluene; at 100 ℃; for 24h; under 60006 Torr; Title compound not separated from byproducts.;
|
|
|
With C24H23Cl3P2Pt; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 24h; under 30003 Torr; optical yield given as %ee; chemoselective reaction; Autoclave; Inert atmosphere;
|
|
|
With (R(P),R(P))-cis-bis(1-propyl-3-methyl-3-phospholano)-dichloro-platinum(II); hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 96h; under 60006 Torr; optical yield given as %ee; regioselective reaction; Inert atmosphere;
|
|
|
With cis-[bis(1-ethyl-3-methyl-3-phospholeno)-dichloro-platinum(II)]; hydrogen; tin(ll) chloride; In toluene; at 60 ℃; for 72h; under 60006 Torr; Reagent/catalyst; Temperature; Time; enantioselective reaction; Inert atmosphere; Autoclave;
|
29 % ee |
|
With PtCl2{(-)-(2S,4S)-2,4-bis(diphenylphosphino)pentane}; hydrogen; tin(ll) chloride; In toluene; at 60 ℃; for 18h; under 60006 Torr; Temperature; enantioselective reaction; Inert atmosphere; Autoclave;
|
40 % ee |
|
With PtCl2{(-)-(2S,4S)-2,4-bis(diphenylphosphino)pentane}; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 3h; under 60006 Torr; Temperature; enantioselective reaction; Inert atmosphere; Autoclave;
|
7 % ee |
|
With cis-[bis((S)-1-isopropyl-3-methyl-3-phospholeno)dichloroplatinum(II)]; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 24h; under 60006 Torr; Temperature; enantioselective reaction; Inert atmosphere; Autoclave;
|
10 % ee |
|
With cis-[bis((R)-4-chloro-1-phenyl-5-methyl-1,2,3,6-tetrahydrophosphinino)-dichloroplatinum(II)]; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 10h; under 60006 Torr; Temperature; Time; chemoselective reaction; Catalytic behavior; Inert atmosphere;
|
8 % ee |
|
With {(R)-binap}PtCl2; hydrogen; tin(ll) chloride; In toluene; at 40 ℃; for 120h; under 30003 Torr; Temperature; enantioselective reaction; Autoclave; Schlenk technique;
|
32 % ee |
|
With {(R)-binap}PtCl2; hydrogen; tin(ll) chloride; In toluene; at 60 ℃; for 120h; under 60006 Torr; enantioselective reaction; Autoclave; Schlenk technique;
|
16 % ee |
|
With {(R)-binap}PtCl2; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 23h; under 30003 Torr; Temperature; enantioselective reaction; Autoclave; Schlenk technique;
|
24 % ee |
|
With {(R)-binap}PtCl2; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 23h; under 60006 Torr; Temperature; enantioselective reaction; Autoclave; Schlenk technique;
|
24 % ee |
|
With (S)-5,5 ′-bis[di(3,5-xylyl)phosphino]-4,4 ′-bi-1,3-benzodioxole; di-μ-chlorobis(norbornadiene)dirhodium(I); hydrogen; at 60 ℃; for 24h; under 60006 Torr; Autoclave; Schlenk technique;
|
|