|
Chemical Composition and Properties
|
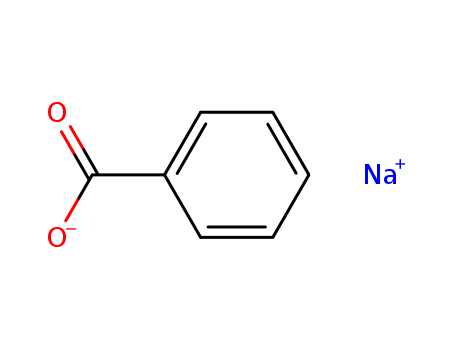
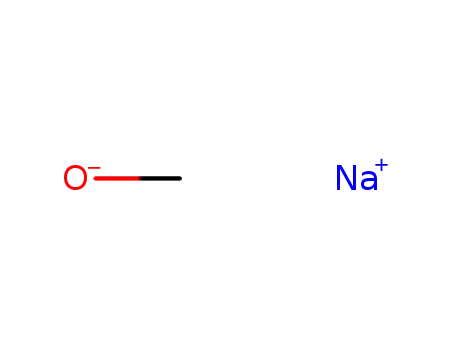
Sodium benzoate is an organic sodium salt derived from the substitution of the proton from the carboxy group of benzoic acid by a sodium ion. It is tasteless, odorless, and soluble in water. Due to its antibacterial and antifungal properties, it is widely utilized as a preservative in food and pharmaceutical products. |
|
Regulatory Approval and Safety
|
Sodium benzoate has been approved as a food preservative by regulatory authorities such as the Food and Drug Administration (FDA). It is generally regarded as safe (GRAS) when consumed in doses of less than 5 mg/kg of body weight per day, with an established Acceptable Daily Intake (ADI). The compound does not accumulate in the body and is metabolized into hippurate, primarily excreted through the urinary system. |
|
Potential Therapeutic Uses
|
Studies suggest that sodium benzoate may have therapeutic potential beyond its preservative function. It has been investigated for its effects on major depressive disorder (MDD), schizophrenia, autism spectrum disorder (ASD), and neurodegenerative diseases. Sodium benzoate has been shown to reduce homocysteine levels, which are associated with MDD and anxiety. Furthermore, it exhibits anti-inflammatory effects and inhibits tryptophan degradation, potentially contributing to the treatment of MDD. |
|
Effects in Animal Studies
|
Sodium benzoate administration in animals has demonstrated effects on lipid profiles, and liver, and kidney parameters. It has been shown to reduce homocysteine levels and exhibit anti-inflammatory effects through modulation of the NF魏B factor. These findings suggest potential therapeutic implications for sodium benzoate in various diseases and conditions. However, further research is needed to fully understand its mechanisms of action and clinical applications. |
|
description
|
Sodium benzoate, also known as benzoic acid sodium, is commonly used as food preservatives in food industry, odorless or with slight smell of benzoin, and tastes sweet astringency. Stable in air, can absorb moisture in open air. It’s naturally found in blueberry, apple, plum, cranberry, prunes, cinnamon and cloves, with weaker antiseptic performance than benzoic acid. Antiseptic performance of 1.180g sodium benzoate is equivalent of about 1g benzoic acid. In acidic environment, sodium benzoate have obvious inhibitory effect on a variety of microorganisms: when pH is at 3.5, 0.05% solution can completely inhibit the growth of yeast; while when pH is above 5.5, it has poor effect on a lot of mold and yeast; hardly has any effect in alkaline solution. After sodium benzoate enters into the body, in the process of biotransformation, it would combine with glycine to be uric acid, or combine with glucuronic acid to be glucosiduronic acid, and all to be eliminated from the body in urine, not to accumulate in the body. As long as it is within the scope of the normal dosage, it would be harmless to the human body, and it is a safe preservatives. It also can be used for carbonated beverages, concentrated juice, margarine, chewing gum base, jam, jelly, soy sauce, etc. Human acceptable daily intake (ADI) < 5 mg/kg body weight (take benzoic acid as calculation basis). Sodium benzoate has big lipophilicity, and it is easy to penetrate cell membrane into the cells, interfere in permeability of cell membrane, and inhibit cell membrane’s absorption of amino acids; cause Ionization acidification of alkaline storage in the cell when entering into, inhibit activity of respiratory enzymes, and stop condensation reaction of acetyl coenzyme A, and thereby achieve the purpose of food antiseptic. The above information is edited by the lookchem He Liaopu. |
|
Chemical properties
|
White crystals or granules, or colorless powder, with sweet astringency. Soluble in water, ethanol, glycerol and methanol. |
|
Content Analysis
|
Take dried sample 1.5g into a 250ml conical flask, dissolve it with 25ml water, and then add 50ml ether and bromophenol. |
|
Toxicity
|
ADI 0~5mg/kg (take benzoic acid as calculation basis, total value of ADI including benzoic acid and its salts and esters; FAO/WHO, 2001).
LD50 4070mg/kg (rats, by oral).
GRAS(FDA,§184.1733,2000). |
|
Production methods
|
1. Neutralized by benzoic acid and sodium bicarbonate. Put water and sodium bicarbonate into the neutralizing pot, boil it and make it dissolved into sodium bicarbonate solution. Mix it with benzoic acid until PH value of the reaction solution reaches to 7-7.5. Heat it to emit over carbon dioxide, and then add active carbon to decolorize it for half an hour. Do suction filtration, after filtrate gets concentrated, put it into flaker tray, dry it to be sheets in the drum, crush it, and then sodium benzoate is made. Consumption rate of benzoic acid (99.5%) 1045kg/t and sodium bicarbonate (98%) 610kg/t.
2. Use 32% soda solution to neutralize benzoic acid in the pot to reach PH value of 7.5, and neutralization temperature is 70℃. Use 0.3% active carbon to decolorize the neutralized solution, vacuum filter it, concentrate, dry it and then it comes to powdered sodium benzoate.
C6H5COOH+Na2CO3→C6H5COONa
3. To get it by toluene oxidation made benzoic acid reacting with sodium bicarbonate, sodium carbonate or sodium hydroxide. |
|
Definition
|
ChEBI: An organic sodium salt resulting from the replacement of the proton from the carboxy group of benzoic acid by a sodium ion. |
|
Production Methods
|
Sodium benzoate is prepared by adding benzoic acid to a hot concentrated solution of sodium carbonate until effervescence ceases. The solution is then evaporated, cooled and allowed to crystallize or evaporate to dryness, and then granulated. |
|
Preparation
|
Produced by the neutralization of benzoic acid with sodium bicarbonate, sodium carbonate or sodium hydroxide. |
|
General Description
|
Sodium benzoate is a sodium salt of benzoic acid, that is freely soluble in water compared to benzoic acid. It is generally used as an antimicrobial preservative in cosmetics, food, and pharmaceuticals.Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. |
|
Hazard
|
Use in foods limited to 0.1%. |
|
Flammability and Explosibility
|
Nonflammable |
|
Pharmaceutical Applications
|
Sodium benzoate is used primarily as an antimicrobial preservative
in cosmetics, foods, and pharmaceuticals. It is used in concentrations
of 0.02–0.5% in oral medicines, 0.5% in parenteral products,
and 0.1–0.5% in cosmetics. The usefulness of sodium benzoate as a
preservative is limited by its effectiveness over a narrow pH range.
Sodium benzoate is used in preference to benzoic acid in some
circumstances, owing to its greater solubility. However, in some
applications it may impart an unpleasant flavor to a product.
Sodium benzoate has also been used as a tablet lubricant at 2–5%
w/w concentrations. Solutions of sodium benzoate have also been administered, orally or intravenously, in order to determine liver
function. |
|
Biochem/physiol Actions
|
Sodium benzoate also has pharmaceutical applications and is component of syrup and transparent tablet. High levels of sodium benzoate may trigger histamine release and also induce cell damage. It is recommended for the treatment of urea cycle disorders. However, high levels of sodium benzoate may contribute to glycine deficiency and may impose neuromodulatory effects. |
|
Safety Profile
|
Poison by subcutaneous and intravenous routes. Moderately toxic by ingestion, intramuscular, and intraperitoneal routes. An experimental teratogen. Experimental reproductive effects. Mutation data reported. Larger doses of 8-10 g by mouth may cause nausea and vomiting. Small doses have little or no effect. Combustible when exposed to heat or flame. When heated to decomposition it emits toxic fumes of Na2O. See also BENZOIC ACID. |
|
Safety
|
Ingested sodium benzoate is conjugated with glycine in the liver to
yield hippuric acid, which is excreted in the urine. Symptoms of systemic benzoate toxicity resemble those of salicylates. Whereas
oral administration of the free-acid form may cause severe gastric
irritation, benzoate salts are well tolerated in large quantities: e.g. 6
g of sodium benzoate in 200mL of water is administered orally as a
liver function test.
Clinical data have indicated that sodium benzoate can produce
nonimmunological contact urtcaria and nonimmunological
immediate contact reactions. However, it is also recognized that
these reactions are strictly cutaneous, and sodium benzoate can
therefore be used safely at concentrations up to 5%. However, this
nonimmunological phenomenon should be considered when
designing formulations for infants and children.
Other adverse effects include anaphylaxis and urticarial
reactions, although a controlled study has shown that the incidence
of urticaria in patients given benzoic acid is no greater than that
with a lactose placebo.
It has been recommended that caffeine and sodium benzoate
injection should not be used in neonates; however, sodium
benzoate has been used by others in the treatment of some neonatal
metabolic disorders. It has been suggested that there is a general
adverse effect of benzoate preservatives on the behavior of 3-yearold
children, which is detectable by parents, but not by a simple
clinical assessment.
The WHO acceptable daily intake of total benzoates, calculated
as benzoic acid, has been estimated at up to 5 mg/kg of bodyweight.
LD50 (mouse, IM): 2.3 g/kg
LD50 (mouse, IV): 1.4 g/kg
LD50 (mouse, oral): 1.6 g/kg
LD50 (rabbit, oral): 2.0 g/kg
LD50 (rat, IV): 1.7 mg/kg
LD50 (rat, oral): 4.1 g/kg |
|
Potential Exposure
|
Sodium benzoate is used as a food and feed additive, flavor, packaging material; pharmaceutical; preservative for food products and tobacco; anti-fungal agent; antiseptic, rust, and mildew inhibitor; intermediate in the manufacture of dyes. Used as a human hygiene biocidal product. |
|
storage
|
Aqueous solutions may be sterilized by autoclaving or filtration. The bulk material should be stored in a well-closed container, in
a cool, dry place. |
|
Shipping
|
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. |
|
Purification Methods
|
Crystallise it from EtOH (12mL/g). [Beilstein 9 IV 27.] |
|
Properties and Applications
|
TEST ITEMS
SPECIFICATION
APPEARANCE
WHITE POWDER
CONTENT OF SODIUM BENZOATE
99.0% min
DRY LOSS
0.10% max
pH VALUE
8
TOTAL CHLORIDE
300 ppm max
TRANSPARENCE
PASS
TOTAL HEAVY METAL
0.001% max
As CONTENT
0.0002% max |
|
TEST ITEMS
|
SPECIFICATION |
|
APPEARANCE
|
WHITE POWDER |
|
CONTENT OF SODIUM BENZOATE
|
99.0% min |
|
pH VALUE
|
8 |
|
TOTAL CHLORIDE
|
300 ppm max |
|
TRANSPARENCE
|
PASS |
|
As CONTENT
|
0.0002% max |
|
Mechanism of food preservation
|
The mechanism starts with the absorption of benzoic acid into the cell. If the intracellular pH changes to 5 or lower, the anaerobic fermentation of glucose through phosphofructokinase is decreased by 95 %, thereby inhibiting the growth and survival of micro-organisms that cause food spoilage. |
|
Incompatibilities
|
Incompatible with quaternary compounds, gelatin, ferric salts,
calcium salts, and salts of heavy metals, including silver, lead, and
mercury. Preservative activity may be reduced by interactions with
kaolin or nonionic surfactants. |
|
Regulatory Status
|
GRAS listed. Accepted as a food additive in Europe. Included in the
FDA Inactive Ingredients Database (dental preparations; IM and IV
injections; oral capsules, solutions and tablets; rectal; and topical
preparations). Included in nonparenteral medicines licensed in the
UK. Included in the Canadian List of Acceptable Non-medicinal
Ingredients. |