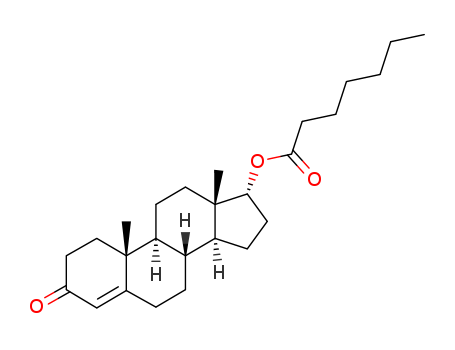
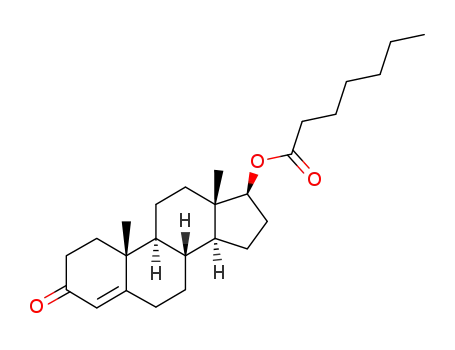
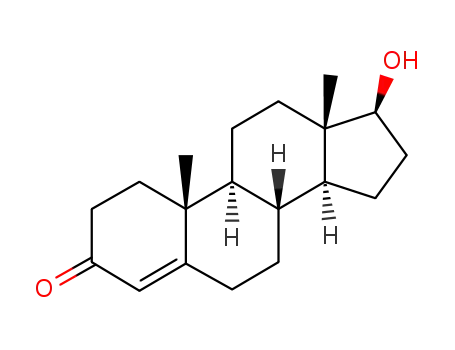
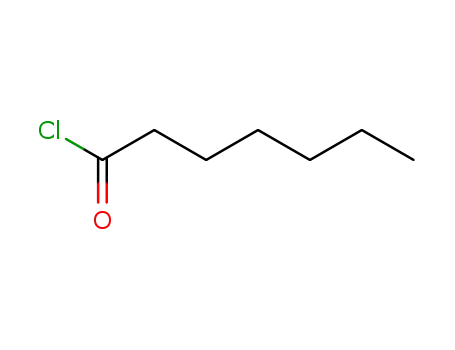
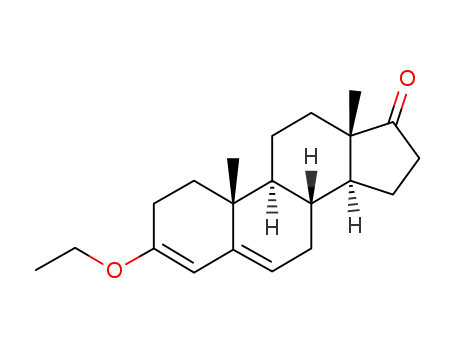
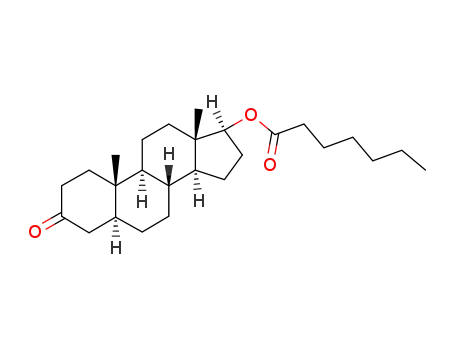
Manufacturer supply Testosterone enanthate 315-37-7 with sufficient stock and high standard
- Molecular Formula:C26H40O3
- Molecular Weight:400.602
- Appearance/Colour:White crystalline powder
- Melting Point:34-39 °C
- Refractive Index:1.4700 (estimate)
- Boiling Point:503.9 °C at 760 mmHg
- Flash Point:214.2 °C
- PSA:43.37000
- Density:1.06 g/cm3
- LogP:6.40050
Testosterone enanthate(Cas 315-37-7) Usage
|
Indications and Usage
|
Testosterone enanthate is a type of androgen drug, and it is a synthetic compound of testosterone proprionate and testosterone enantate. Its effects are the same as those of testosterone acetate, which include promoting the development of male sex organs and secondary sex characteristics and resisting estrogen. It also has protein assimilating effects, which allow it to help muscle growth and weight gain. Testosterone enanthate can inhibit the pituitary gland and promote sex hormone secretion, thus decreasing the endogenous testosterone secreted by Leydig cells, which achieves the purpose of stopping spermatogenesis.
Testosterone enanthate can be used as a long-term male birth control drug. It is clinically used to treat male hypogonadism and gonadal insufficiency, sex organ hypogenesis, infertility, eunuchism, and cryptorchidism. It can also be used to treat female dysfunctional uterine bleeding, menopausal syndrome, breast cancer and uterine cancer. It can also treat cirrhosis, aplastic anemia, osteoporosis and other consumptive diseases. |
|
Drug Metabolism
|
After injection, it creates a blood concentration higher than the physiological level, and then blood concentration decreases rapidly. |
|
Clinical Research
|
In a trial that injected monkeys with testosterone enanthate every week, the monkeys’ sperm cells began experiencing apoptosis in a week, with apoptosis rates peaking in the fifth week. A clinical trial with 308 participants also showed that testosterone enanthate’s birth-control effects are much more effective on Asian men than on Caucasian men, as over 95% of Asian men achieved azoospermia, while only 40-70% of Caucasian men did.
A trial of over 100 volunteers showed that weekly muscle injections of 250mg led to most men developing azoospermia or severe ogliospermia in 10 weeks, and that sperm count could recover after ceasing injection for a certain period of time. If the injection intervals are extended to over 10-12 days, even increasing dosage will not have desired effects. Testosterone enanthate only needs to be injected once a week, which is an advantage over testosterone acetate. |
|
Adverse reactions
|
Large dosages or extended use can cause water and sodium retention and edema. Young women may exhibit virilism, with characteristics including deepened voice, hair growth, acne, clitoral hypertrophy, etc. Not to be used by patients with prostatic cancer or pregnant patients. Should be used with caution by patients with prostate hypertrophy and liver and kidney dysfunction. When treating breast cancer, drug usage should be stopped immediately if hypercalcemia is detected. |
|
Manufacturing Process
|
A mixture of testosterone, pyridine and oenanthic acid anhydride is heated for
1 1/2 hours to 125°C. The cooled reaction mixture is decomposed with water
while stirring and cooling. After prolonged standing at a temperature below
room temperature, the whole is extracted with ether and the ethereal solution
is washed consecutively with dilute sulfuric acid, water, 5% sodium hydroxide
solution, and again with water. The crude ester remaining on evaporation of
the dried ether solution, after recrystallization from pentane, melts at 36° to
37.5°C. |
|
Therapeutic Function
|
Androgen |
|
Definition
|
ChEBI: Testosterone enanthate is a heptanoate ester and a sterol ester. It has a role as an androgen. It is functionally related to a testosterone. |
InChI:InChI=1/C19H28O.C7H14O2/c1-18-9-3-4-16(18)15-6-5-13-12-14(20)7-11-19(13,2)17(15)8-10-18;1-2-3-4-5-6-7(8)9/h12,15-17H,3-11H2,1-2H3;2-6H2,1H3,(H,8,9)/p-1/t15-,16-,17-,18-,19-;/m0./s1
315-37-7 Relevant articles
Preparation method of alkyl acid testosterone
-
Paragraph 0022-0023, (2020/11/10)
The invention discloses a preparation me...
Synthesis method of alkyl acid testosterone
-
, (2020/12/10)
The invention discloses a synthesis meth...
Long-range effect of 17-substituents in 3-oxo steroids on 4,5-double bond hydrogenation
Sidova, Romana,Stransky, Karel,Kasal, Alexander,Slavikova, Barbora,Kohout, Ladislav
, p. 1528 - 1542 (2007/10/03)
The long-range effect of substituents in...
315-37-7 Process route
-
-
26614-48-2,165304-83-6
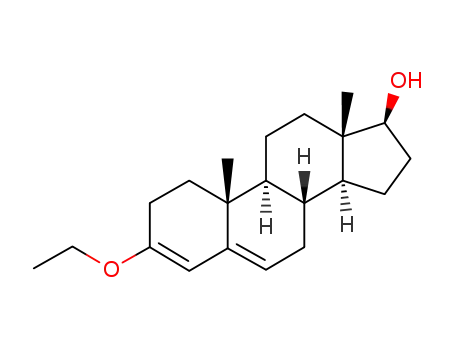
17β-hydroxy-3-ethoxyandrosta-3,5-diene
-
-
315-37-7
testosterone heptanoate
Conditions
| Conditions |
Yield |
|
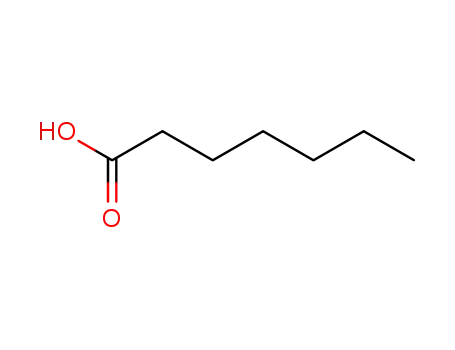
oenanthic acid; 17β-hydroxy-3-ethoxyandrosta-3,5-diene;
With
dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;
In
1,2-dichloro-ethane;
at 20 ℃;
Inert atmosphere;
With
sulfuric acid;
In
water;
|
96.3%
|
-
-
315-37-7
testosterone heptanoate
Conditions
| Conditions |
Yield |
|
oenanthic acid; testosterone;
With
dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;
In
1,2-dichloro-ethane;
at 20 ℃;
Inert atmosphere;
With
sulfuric acid;
In
water;
|
89.6%
|
315-37-7 Upstream products
-
58-22-0
testosterone
-
2528-61-2
Heptanoic acid chloride
-
972-46-3
3-ethoxyandrosta-3,5-dien-17-one
-
26614-48-2
17β-hydroxy-3-ethoxyandrosta-3,5-diene
315-37-7 Downstream products