|
Indications
|
Alleviate the acute phase of various kinds of chronic arthritis such as rheumatoid arthritis, osteoarthritis, spondyloarthropathies, gouty arthritis and rheumatoid arthritis as well as persistent symptoms of joint swelling and pain. It can be used for the non-cause treatment and control of disease.
For the treatment of various kinds of non-joint soft tissue rheumatic pain, such as shoulder pain, tenosynovitis, bursitis, myalgia and post-exercise pain.
For the treatment of acute mild to moderate pain such as: post-surgery, post-trauma, post-strain, primary dysmenorrhea, toothache, headache and so on.
It has an antipyretic effect against the fever of adults and children. |
|
Used in Particular Diseases
|
Acute Gouty Arthritis:
Dosage and Frequency:?800 mg four times a day |
|
Increase stroke risk
|
Ibuprofen is one of the most commonly used non-prescription painkillers, commonly used in the treatment of arthritis, muscle pain, neuralgia, headache, migraine, toothache, dysmenorrhea or low back pain. A recent study published in the British Medical Journal found that people who have taken a large number of antipyretic drugs, ibuprofen, have a 3-fold increase in the risk of getting stroke or heart disease.
Researchers from the University of Berne in Switzerland reviewed 31 clinical trials involving more than 11.6 million patients. Patients were treated with one of seven common analgesics. The results showed that patients subjecting to long-term administration of large doses of ibuprofen not only have a risk of getting stroke increased by 3 times, but also have significantly increased risk of suffering heart attack and heart disease death. However, the study also showed that occasionally taking ibuprofen for the treatment of headache will not be dangerous. The study also found that commonly used analgesic diclofenac sodium also has a similar problem.
The study found a health risk associated with long-term use of ibuprofen, being similar to the anti-arthritis drug rofecoxib (Velcro), which was halted in 2004 due to safety concerns. |
|
Precautions
|
1.For late pregnancy women, it can prolong the pregnancy, causing dystocia and prolonged pregnancy course. Pregnant women and lactating women should not administrate it.
2. Inhibition of platelet aggregation; it can extent the bleeding time. This effect will disappear at 24 hours after withdrawal of the drug.
3. It can increase the blood urea nitrogen and serum creatinine content, further reducing the creatinine clearance rate. The following circumstances should be used with caution:
Bronchial asthma can be aggravated after treatment.
Heart failure, high blood pressure; medication can cause water retention, edema.
Hemophilia or other hemorrhagic diseases (including coagulation disorders and platelet dysfunction); medication can cause prolonged bleeding time, increase the bleeding tendency.
Patients with a history of gastrointestinal ulcers are prone to get gastrointestinal side effects, including generating new ulcers.
Patients of renal dysfunction, after administration, can get increased renal adverse reactions, and even get renal failure.
During long-term medication, it should be regularly checked of blood phase and liver, kidney function. |
|
Drug Interactions
|
Drinking or combination with other non-steroidal anti-inflammatory drugs can increase the gastrointestinal side effects, and have the risk of ulcers. Long-term combination with acetaminophen can increase the toxic side effects on the kidney.
Combination with aspirin or other salicylic acid drugs causes no increase in the efficacy, but cam cause gastrointestinal adverse reactions and increase of the bleeding tendency.
Combination with heparin, dicoumarol and other anticoagulants as well as platelet aggregation inhibitors has the risk for increasing bleeding.
Combination with furosemide can weaken the sodium excretion effect and antihypertensive effect.
Combination with verapamil and nifedipine can increase the plasma concentration of the product.
Ibuprofen can increase the plasma concentration of digoxin; pay attention to adjusting the dose of digoxin upon co-administration.
Ibuprofen can enhance the role of anti-diabetic drugs (including oral hypoglycemic agents).
The goods, when used in combination with antihypertensive drugs can affect the antihypertensive effect of the latter one.
Probenecid can reduce the excretion of the goods, increase the concentration of blood, thereby increasing the toxicity, so it is proper to reduce the dosage upon co-administration.
The goods can reduce the excretion of methotrexate, increase the blood concentration which can reach up to the level of poisoning, so the goods should not be used with medium or large doses of methotrexate. |
|
Side Effects
|
Gastrointestinal symptoms include indigestion, stomach burning sensation, stomach pain and nausea as well as vomiting. This usually appears in 16% long-term administrators. These symptoms will disappear upon drug withdraw. In most cases, the patients can tolerate even without withdrawal. A small number (<1%) of patients can get gastric ulcer and gastrointestinal bleeding. This are also cases of perforation due to ulcer.
Neurological symptoms such as headache, lethargy and dizziness; Tinnitus (rare) appears in 1% to 3% of patients.
Renal insufficiency is rare, mostly occur in patients of potential kidney disease; but a small number of patients may obtain lower extremity edema.
Other rare symptoms also include rash, bronchial asthma attack, elevated liver enzymes and leukopenia.
During medication, there might be emergence of gastrointestinal bleeding, liver and kidney dysfunction, visual impairment, abnormal blood and allergic reactions, etc., that should be discontinued. |
|
History
|
Ibuprofen
was developed while searching for an alternative pain reliever to aspirin in the 1950s. It and
related compounds were synthesized in 1961 by Stewart Adams, John Nicholson, and Colin
Burrows who were working for the Boots Pure Drug Company in Great Britain. Adams and
Nicholson filed for a British patent on ibuprofen in 1962 and obtained the patent in 1964;
subsequent patents were obtained in the United States. The patent of Adams and Nicholson
was for the invention of phenylalkane derivatives of the form shown in Figure 49.1, where
R1 could be various alkyl groups, R2 was hydrogen or methyl, and X was COOH or COOR,
with R being alkyl or aminoalkyl groups. The first clinical trials for ibuprofen were started in
1966. Ibuprofen was introduced under the trade name Brufen in 1969 in Great Britain. It was
introduced in the United States in 1974. Ibuprofen was initially off ered by prescription, but
it became available in over-the-counter medications in the 1980s. |
|
Manufacturing Process
|
Isobutylbenzene is first acetylated to give isobutylacetophenone. 4-i�butylacetophenone (40 g), sulfur (11 g) and morpholine (30 ml) were refluxed
for 16 hours, cooled, acetic acid (170 ml) and concentrated hydrochloric acid
(280 ml) were added and the mixture was refluxed for a further 7 hours. The mixture was concentrated in vacuo to remove acetic acid and the concentrate
was diluted with water.The oil which separated was isolated with ether, the ethereal solution was
extracted with aqueous sodium carbonate and this extract was acidified with
hydrochloric acid. The oil was isolated with ether, evaporated to dryness and
the residue was esterified by refluxing with ethanol (100 ml) and concentrated
sulfuric acid (3 ml) for 5 hours. The excess alcohol was distilled off, the
residue was diluted with water, and the oil which separated was isolated with
ether. The ethereal solution was washed with sodium carbonate solution; then
with water and was dried. The ether was evaporated off and the oil was
distilled to give ethyl 4-i-butylphenylacetate.Sodium ethoxide from sodium (3.67 g) in absolute alcohol (64 ml) was added
over 20 minutes with stirring to a mixture of ethyl 4-i-butylphenylacetate
(28.14 g) and ethyl carbonate (102 ml) at 100°C. The reaction flask was
fitted with a Fenske column through which alcohol and then ethyl carbonate
distilled. After 1 hour when the still head reached 124°C heating was
discontinued. Glacial acetic acid (12 ml) and water (50 ml) was added to the
stirred ice-cooled mixture and the ester isolated in ether, washed with sodium
carbonate solution, water and distilled to give ethyl 4-i-butylphenylmalonate.Ethyl 4-i-butylphenylmalonate (27.53 g) in absolute alcohol (25 ml) was
added with stirring to a solution of sodium ethoxide From sodium (2.17 g) in
absolute alcohol (75 ml). Ethyl iodide (15 ml) was added and the mixture
refluxed for 2% hours, the alcohol distilled and the residue diluted with water,
extracted with ether, washed with sodium bisulfite, water, and evaporated to
dryness.The residual oil was stirred and refluxed with sodium hydroxide (75 ml of 5
N), water (45 ml) and 95% ethanol (120 ml). Within a few minutes a sodium
salt separated and after 1 hour the solid was collected, washed with ethanol,
dissolved in hot water and acidified with dilute hydrochloric acid to give the
methyl malonic acid which was collected and dried in vacuo MP 177° to 180°C
(dec.).The malonic acid (9 g) was heated to 210° to 220°C in an oil bath for 20
minutes until decarboxylation had ceased. The propionic acid was cooled and
recrystallized from light petroleum (BP 60° to 80°C). Two further
recrystallizations from the same solvent gave colorless prisms of 2-(4-
isobutylphenyl)propionicacid MP 75° to 77.5°C. (The procedure was reported
in US Patent 3,228,831.) |
|
Therapeutic Function
|
Antiinflammatory |
|
World Health Organization (WHO)
|
Ibuprofen, a non-steroidal anti-inflammatory agent, was
introduced in 1969. It was approved for sale without prescription in packages
containing no more than 400 mg, in the United Kingdom in 1983. This action was
followed by the USA, Canada and several European countries. Since this time
reports of suspected adverse effects have increased. Most of these relate to gastrointestinal
disturbances, hypersensitivity reactions but aseptic meningitis, skin
rashes and renal damage have been recorded. |
|
Synthesis Reference(s)
|
Chemical and Pharmaceutical Bulletin, 31, p. 3139, 1983 DOI: 10.1248/cpb.31.3139The Journal of Organic Chemistry, 52, p. 287, 1987 DOI: 10.1021/jo00378a027 |
|
Flammability and Explosibility
|
Nonflammable |
|
Biochem/physiol Actions
|
Primary TargetCOX-1 |
|
Pharmacokinetics
|
Ibuprofen is rapidly absorbed on oral administration, with peak plasma levels being generally attained within 2 hours
and a duration of action of less than 6 hours. As with most of these acidic NSAIDs, ibuprofen (pKa = 4.4) is
extensively bound to plasma proteins (99%) and will interact with other acidic drugs that are protein bound. |
|
Synthesis
|
Ibuprofen, 2-(4-iso-butylphenyl)propionic acid (3.2.23), can be synthesized
by various methods [88–98]. The simplest way to synthesize ibuprofen is by the acylation
of iso-butylbenzol by acetyl chloride. The resulting iso-butylbenzophenone (3.2.21) is
reacted with sodium cyanide, giving oxynitrile (3.2.22), which upon reaction with
hydroiodic acid in the presence of phosphorus is converted into 2-(4-iso-butylphenyl)pro�pionic acid (3.2.23), which subsequently undergoes phases of dehydration, reduction, and
hydrolysis.Another way to synthesize ibuprofen consists of the chloromethylation of iso-butylbenzene, giving 4-iso-butylbenzylchloride (3.2.24). This product is reacted with sodium
cyanide, making 4-iso-butylbenzyl cyanide (3.2.25), which is alkylated in the presence of
sodium amide by methyl iodide into 2-(4-iso-butylbenzyl)propionitrile (3.2.26).
Hydrolysis of the resulting product in the presence of a base produces ibuprofen (3.2.23). |
|
Drug interactions
|
Potentially hazardous interactions with other drugs
ACE inhibitors and angiotensin-II antagonists:
antagonism of hypotensive effect; increased risk of
nephrotoxicity and hyperkalaemia. Analgesics: avoid concomitant use of 2 or more
NSAIDs, including aspirin (increased side effects);
avoid with ketorolac (increased risk of side effects
and haemorrhage); possibly reduced antiplatelet
effect with aspirin.
Antibacterials: possibly increased risk of convulsions
with quinolones.
Anticoagulants: effects of coumarins and
phenindione enhanced; possibly increased risk of
bleeding with heparins, dabigatran and edoxaban -
avoid long term use with edoxaban.
Antidepressants: increased risk of bleeding with
SSRIs and venlaflaxine.
Antidiabetic agents: effects of sulphonylureas
enhanced.
Antiepileptics: possibly increased phenytoin
concentration.
Antivirals: increased risk of haematological toxicity
with zidovudine; concentration possibly increased by
ritonavir.
Ciclosporin: may potentiate nephrotoxicity.
Cytotoxics: reduced excretion of methotrexate;
increased risk of bleeding with erlotinib.
Diuretics: increased risk of nephrotoxicity;
antagonism of diuretic effect; hyperkalaemia with
potassium-sparing diuretics.
Lithium: excretion decreased.
Pentoxifylline: increased risk of bleeding.
Tacrolimus: increased risk of nephrotoxicity. |
|
Environmental Fate
|
Ibuprofen has a high water solubility and low volatility, which
suggest a high mobility in the aquatic environment. This makes
it a commonly detected chemical of the pharmaceutical and
personal care products (PPCPs) in the environment. It is not as
persistent, however, as many other chemicals. Ibuprofen
undergoes photodegradation with exposure to direct and
indirect sunlight, although degradation products can have
effects on aquatic environments. |
|
Metabolism
|
Metabolism occurs rapidly, and the drug is nearly completely excreted in the urine as unchanged drug and oxidative
metabolites within 24 hours following administration. Metabolism by CYP2C9 (90%) and CYP2C19 (10%)
involves primarily ω-, and ω1-, and ω2-oxidation of the p-isobutyl side chain, followed by alcohol oxidation of the primary alcohol resulting from ω–oxidation to the corresponding carboxylic acid. All metabolites are inactive. When
ibuprofen is administered as the individual enantiomers, the major metabolite isolated is the S-(+)-enantiomer
whatever the configuration of the starting enantiomer. Interestingly, the R-(–)-enantiomer is inverted to the
S-(+)-enantiomer in vivo via an acetyl–coenzyme A intermediate, accounting for the observation that the two
enantiomers are bioequivalent in vivo. This is a metabolic phenomenon that also has been observed for other
arylpropionic acids, such as ketoprofen, benoxaprofen, fenoprofen, and naproxen. |
|
Toxicity evaluation
|
The mechanisms of ibuprofen-induced toxicity have not been
clearly defined. Acute renal failure is postulated to result from
decreased production of intrarenal prostaglandins via inhibition
of the cyclooxygenase pathway. In turn, this will decrease
the renal blood flow and glomerular filtration rate. Ibuprofen
also interferes with prostaglandin synthesis in the gastrointestinal
system, which can contribute to its irritating effect on the
mucosa of the gastrointestinal tract. Anion gap metabolic
acidosis is likely caused by elevated lactate due to hypotension
and hypoperfusion and also due to ibuprofen and its metabolites,
which are all weak acids. Seizures have been reported in
large ibuprofen overdoses, but the mechanism of toxicity
remains unknown. In massive overdoses, ibuprofen is thought
to have cellular toxicity disrupting mitochondrial energy
processes causing the formation of lactic acid. |
|
references
|
[1]. kato m, nishida s, kitasato h, et al. cyclooxygenase-1 and cyclooxygenase-2 selectivity of non-steroidal anti-inflammatory drugs: investigation using human peripheral monocytes. j pharm pharmacol, 2001, 53(12): 1679-1685.[2]. janssen a, schiffmann s, birod k, et al. p53 is important for the anti-proliferative effect of ibuprofen in colon carcinoma cells. biochem biophys res commun, 2008, 365(4): 698-703.[3]. dabhi jk, solanki jk, mehta a. antiatherosclerotic activity of ibuprofen, a non-selective cox inhibitor--an animal study. indian j exp biol, 2008, 46(6): 476-481.[4]. redondo-castro e, navarro x. chronic ibuprofen administration reduces neuropathic pain but does not exert neuroprotection after spinal cord injury in adult rats. exp neurol, 2014, 252: 95-103. |
|
Chemical Structure and Properties
|
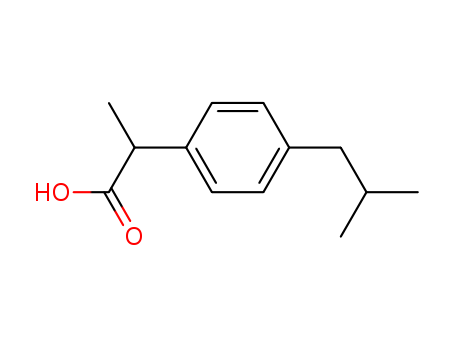
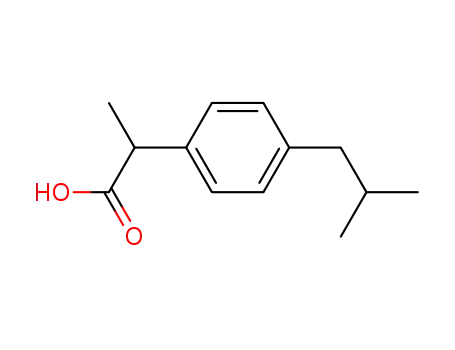
Ibuprofen is a monocarboxylic acid derived from propionic acid, with one of the hydrogens substituted by a 4-(2-methylpropyl)phenyl group.
It is a colorless, crystalline solid with a characteristic odor. Ibuprofen is a non-steroidal anti-inflammatory drug (NSAID) used for the treatment of fever, muscle pain, and inflammation.
Its discovery dates back to 1961 by Stewart Adams, and it remains one of the most prescribed NSAIDs today. |
|
History and Market Presence
|
Initially introduced for prescription use in the UK in 1969 (as Brufen) and in the US in 1974, ibuprofen later transitioned to over-the-counter (OTC) sales in both countries in the 1980s. It gained popularity for various pain complaints, including headaches. |
|
Usage and Consumption
|
Ibuprofen is rapidly absorbed by the body but has a short half-life, requiring frequent dosing. It belongs to the group of NSAIDs and is the third most consumed drug worldwide, with a significant annual output.
Ibuprofen is listed as a priority substance by regulatory bodies due to its environmental impact. |
|
Pharmacokinetics and Metabolism
|
The therapeutic doses of ibuprofen range from 600 to 1200mg/day. After oral administration, ibuprofen primarily binds to plasma albumin and undergoes metabolism to several metabolites, which are eventually excreted. |
|
Formulation and Delivery
|
Traditional formulations of ibuprofen have poor water solubility, necessitating higher doses for therapeutic effect.
The development of a water-soluble form of ibuprofen is essential, especially for targeting high concentrations in the lungs using inhalation devices. |
|
Therapeutic Potential Beyond Pain Relief
|
In silico predictions suggest ibuprofen's potential as an entry inhibitor for viruses like Ebola and Zika, with experimental confirmations of its virucidal effects. Ibuprofen has also shown promise in the treatment of patent ductus arteriosus (PDA) in preterm infants by inhibiting cyclooxygenase. |
|
description
|
Ibuprofen belongs to a non-steroidal anti-inflammatory analgesic. It has excellent anti-inflammatory, analgesic and antipyretic effect with less adverse reactions. It has been widely used in the world, as the world's best-selling non-prescription drugs. It, together with aspirin and paracetamol are listed as the three key antipyretic analgesics products. In our country, it is mainly used in pain alleviation and anti-rheumatism, etc. It has much less applications in the treatment of cold and fever compared with paracetamol and aspirin. There are a dozens of pharmaceutical companies qualified for production of ibuprofen in China. But the bulk of the domestic market sales of ibuprofen have been occupied by Tianjin Sino-US Company.
The Ibuprofen was co-discovered by Dr. Stewart Adams (later he becomes a professor and won the Medal of the British Empire) and his team including CoLinBurrows and Dr. John Nicholson. The aim of the initial study was to develop a "super aspirin" to obtain an alternative for the treatment of rheumatoid arthritis that is comparable to that of aspirin but with less serious adverse reactions. For other drugs such as phenylbutazone, it has a high risk of causing adrenal suppression and other adverse events such as gastrointestinal ulcers. Adams decided to look for a drug with good gastrointestinal resistance, which is particularly important for all non-steroidal anti-inflammatory drugs.
Phenyl acetate drugs have aroused people's interest. Although some of these drugs have been found to be at risk of causing ulcers based on the dog's test, Adams is aware of that this phenomenon may be due to a relatively long half-life of the drug clearance. In this class of drugs there is a compound – ibuprofen, which has a relatively short half-life, sustaining only 2 hours. Among the screened alternative drugs, although it is not the most effective, it is the most secure. In 1964, ibuprofen had become the most promising alternative to aspirin. |
|
Definition
|
ChEBI: A monocarboxylic acid that is propionic acid in which one of the hydrogens at position 2 is substituted by a 4-(2-methylpropyl)phenyl group. |
|
Brand name
|
Abbifen;Abuprohm;Abu-tab;Aches-n-pain;Acril;Actifen;Actiprofen;Actren;Addaprin;Advil 200 mg;Advil cold & sinus;Agisan;Aktren;Aldospray;Algiasdin;Algifor;Algisan;Algofer;Altior;Amersol;Anadin ibuprofen;Analgesico;Analgil;Analgyl;Anco;Antalgil;Antiflam;Antiruggen;Apsifen;Artofen;Artren;Artril;Artrofen;Bayer select ibuprofen pain reliever;Benflogin;Betagesic;Betaprofen;Brofen 200 mg;Brofen 400 mg;Brufert;Brufort;Buborone;Bufedon;Bufigen;Burana;Cesra;Children's advil;Children's motrin;Codafen continus;Contraneural;Contrneural;Cuisialigil;Cunil;Cuprofen;Dansida;Dentigoa forte;Dignoflex;Dimetap sinus;Dimidon;Dismenodl n;Dolgirit;Dolocyl;Dolo-dolgit;Dologesic;Dolo-neos;Dolo-puren;Doltibil;Dolven;Donjust-b;Dorival;Dristan sinus;Duradyne;Dura-ibu;Duralbuprofen;Dysdolen;Ecoprofen;Ediluna;Esprenit;Excedrin ib;Exidol;Exneural;Femafen;Femapirin;Femidol;Fenalgic;Fenlong;Genpril;Guildprofen;Halprin;Ibenon;Ibol;Ibosure;Ibruthalal;Ibu-attritin;Ibucasen;Ibu-cream;Ibufac;Ibufen tablets;Ibufen-l;Ibufug;Ibugel;Ibugesic;Ibuhexal;Ibular;Ibulav;Ibuleve;Ibulgan;Ibumetin;Ibuphlogont;Ibupirac;Ibuprin;Ibuprofen 200;Ibuprohm;Ibu-slow;Ibusure;Ibu-tab;Ibutad;Ibutid;Ibutop;Ibuvivimed;Ibux;Imben;Inabrin;Incefal;Inflam;Inoven;Inza;Iproben;Irfen;Isdol;Isisfen;Junifen;Kalma;Kos;Lacondan;Librofem;Librofen;Lidifen;Lisi-budol;Mediprofen;Melfen;Menado ibuprofen usp;Midol 200 advanced pain formula;Midol ib;Migrafen;Minadol;Moment;Motrin ib;Narfen;Neobrofen;Neobrufen;Nerofen;Niapren;Novaprin;Novogent;Novoprofen;Nu-ibuprofen;Optifen;Opturem;Pacifene;Padudent;Paxofen;Pfeil;Phor pain;Posodolor;Prontalgin;Recudik;Relcofen;Rheufen;Rimafen;Saleto-600;Seclodin;Sedaspray;Serviprofen;Sine-aid ib;Solufen;Spedifen;Stadasan;Superior pain medicine;Supreme pain medicine;Supren;Suspren;Tabalon;Tempil;Tendar;Trauma-dolgit;Ultraprin;Valprin. |
|
General Description
|
Ibuprofen, 2-(4-isobutylphenyl)propionic acid (Motrin,Advil, Nuprin), was introduced into clinical practice followingextensive clinical trials. It appears to have comparableefficacy to aspirin in the treatment of RA, but with a lowerincidence of side effects. It has also been approved for usein the treatment of primary dysmenorrhea, which is thoughtto be caused by an excessive concentration of PGs and endoperoxides. However, a recent study indicates that concurrentuse of ibuprofen and aspirin may actually interferewith the cardioprotective effects of aspirin, at least in patientswith established cardiovascular disease. This is becauseibuprofen can reversibly bind to the platelet COX-1isozymes, thereby blocking aspirin’s ability to inhibit TXA2synthesis in platelets. |