|
Chemical Description
|
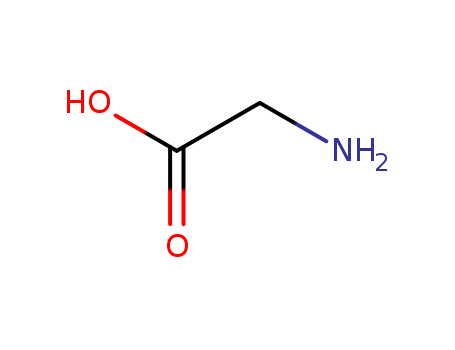
Glycine is the simplest amino acid, with a hydrogen atom as its side chain. |
|
Advantages and Environmental Impact
|
Glycine is preferred over other amino acids due to its low cost, bulk availability, environmental safety, stability, and biodegradability. It offers advantages over conventional leaching agents in various applications. |
|
Synthesis and Metabolism
|
While considered non-essential, glycine can be synthesized endogenously from sources such as serine, choline, and hydroxyproline. Key enzymes involved in glycine synthesis and metabolism include serine hydroxymethyltransferase, peroxisomal sarcosine oxidase, and alanine--glyoxylate aminotransferase. |
|
Role in Neurotransmission
|
Glycine acts as a co-agonist for N-methyl-D-aspartate (NMDA) receptors, playing integral roles in synaptic plasticity and memory function. Dysfunction in the NMDA receptor system may contribute to age-related decline in learning and memory. |
|
Clinical Studies and Effects on Memory
|
Clinical studies have reported mixed results regarding the effects of glycine on memory enhancement. While some trials have shown improvements in memory function, others have yielded inconclusive findings. |
|
Diversity and Behavior
|
Despite its simplicity, glycine exhibits endless diversity in behavior across various phenomena. It was the first amino acid for which polymorphism was reported, and its polymorphs differ in physical properties and biological activity. Glycine clusters persist in solution, leading to "solution memory." |
|
Amino acids with the simplest structure
|
Glycine is of the simplest structure in the 20 members of amino acid series, also known as amino acetate. It is a non-essential amino acid for the human body and contains both acidic and basic functional group inside its molecule. It exhibits as a strong electrolyte an aqueous solution, and has a large solubility in strong polar solvents but almost insoluble in non-polar solvents. Moreover, it also has a relative high melting point and boiling point. The adjustment of the pH of the aqueous solution can make glycine exhibit different molecular forms. The side chain of glycine contains only a hydrogen atom. Owing to another hydrogen atom connecting to the α-carbon atom, the glycine is not optical isomer. Since the side bond of glycine is very small, it can occupy space which can’t be occupied by other amino acids, such as those amino acids located within the collagen helix. At room temperature, it exhibits as white crystal or light yellow crystalline powder and has a unique sweet taste which can ease the taste of acid and alkaline taste, masking the bitter taste of saccharin in food and enhance the sweetness. However, if an excessive amount of glycine is absorbed by body, they not only can’t be totally absorbed by the body, but will also break the balance of the body's absorption of amino acids as well as affect the absorption of other kinds of amino acids, leading to nutrient imbalances and negatively affected health. The milk drink with glycine being the major raw material can easily does harm to the normal growth and development of young people and children. It has a density of 1.1607, melting point of 232~236 °C (decomposition). It is soluble in water but insoluble in alcohol and ether. It is capable of acting together with hydrochloric acid to form hydrochloride salt. It is presented in the muscles of animals. IT can be produced from the reaction between monochloro acetate and ammonium hydroxide as well as from the hydrolysis of gelation with further refining. |
|
History of discovery
|
Amino acids are organic acids containing an amino group and are the basic units of protein. They are generally colorless crystals with a relative high melting point (over 200 °C). It is soluble in water with amphiprotic ionization characteristics and can have sensitive colorimetric reaction with ninhydrin reagent. In 1820, glycine with the simplest structure was first discovered in a protein hydrolysis product. Until 1940, it has been found that there were about 20 kinds of amino acids in nature. They are necessary for the protein synthesis of both human and animal. They are mostly α-L-type amino acids. According to the different number of amino groups and carboxyl groups contained in amino acids, we classify amino acids into neutral amino acids (glycine, alanine, leucine, isoleucine, valine, cystine, cysteine, A methionine, threonine, serine, phenylalanine, tyrosine, tryptophan, proline and hydroxyproline, etc.) with the amino acid molecules containing only one amino group and a carboxyl group; acidic amino acid (glutamate, aspartate) which contains two carboxyl and one amino group; alkaline amino acids (lysine, arginine) which molecularly contains one carboxyl group and two amino groups; Histidine contains a nitrogen ring which exhibits weakly alkaline and thus also belonging to alkaline amino acids. Amino acids can be obtained both from protein hydrolysis and from chemical synthesis. Since the 1960s, industrial production mainly applied microbial fermentation, such as monosodium glutamate factory has been widely applied fermentation method for production of glutamate. In recent years, people has also applied petroleum hydrocarbons and other chemical products as raw materials of fermentation for production of amino acids.
The above information is edited by the lookchem of Dai Xiongfeng. |
|
Content Analysis
|
Accurately weigh 175 mg of sample which has undergone drying for 2 h at 105 °C and place it in a 250m1 flask, add 50 mL of glacial acetic acid for dissolving; add 2 drops of crystal violet test solution (TS-74); titrate with 0.1ml/L perchloric acid to blue-green endpoint. At the same time carry out a blank test, and make the necessary corrections. Each mL of 0.1mol/L perchloric acid is equivalent to glycine (C2H5NO2) 7.507mg. |
|
Biosynthesis of glycine production
|
In the late 1980s, Japan's Mitsubishi Corporation added the screened aerobic Agrobacterium, Brevibacterium, Corynebacterium genus to the medium containing carbon, nitrogen and inorganic nutrient solution for cultivation, and then applied this class of bacteria for converting ethanolamine to glycine in 25~45 °C and pH value from 4 to 9 and further applied concentration, neutralization ion exchange treatment to get the glycine product.
After entering the 1990s, there had been new progress on the production technology of glycine in foreign countries. The Nitto Chemical Industry Co (Japan) add cultured pseudomonas genus, casein bacteria genus, and alcaligenes genus and other species in 0.5% (mass fraction, dry weight) to the glycine amine-containing matrix for reaction of 45 h under 30 °C and pH value of 7.9 to 8.1 with almost all glycine amine being hydrolyzed into glycine with the conversion rate of 99%. Although biological methods are still in the research stage, however, owing to its high selectivity, non-pollution property, it will be a synthetic route with highly development potential. |
|
Production Methods
|
Glycine was discovered in 1820, by Henri Braconnot who boiled gelatin with sulfuric acid. Glycine is manufactured industrially by treating chloroacetic acid with ammonia : ClCH2COOH + 2 NH3→H2NCH2COOH + NH4Cl About 15 million kg are produced annually in this way. In the USA (by GEO Specialty Chemicals, Inc.) and in Japan (by Shoadenko), glycine is produced via the Strecker amino acid synthesis. |
|
Preparation
|
From chloroacetic acid and ammonia; from protein sources, such as gelatin and silk fbroin; from ammonium bicarbonate and sodium cyanide; by catalytic cleavage of serine; from hydrobromic acid and methyleneaminoacetonitrile. |
|
Definition
|
ChEBI: The simplest (and the only achiral) proteinogenic amino acid, with a hydrogen atom as its side chain. |
|
Biosynthesis
|
Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine, which is in turn derived from 3-phospho glycerate. In most organisms, the enzyme Serine hydroxy methyl transferase catalyses this transformation via the cofactor pyridoxal phosphate : serine + tetra hydro folate → glycine +N5,N10-Methylene tetrahydrofolate + H2O In the liver of vertebrates, glycine synthesis is catalyzed by glycine synthase (also called glycine cleavage enzyme). This conversion is readily reversible : CO2 + NH4+ + N5,N10-Methylene tetra hydro folate + NADH + H+→ Glycine + tetrahydrofolate +NAD+Glycine is coded by codons GGU, GGC, GGA and GGG. Most proteins incorporate only small quantities of glycine. A notable exception is collagen, which contains about 35 % glycine. |
|
Biotechnological Production
|
Glycine is manufactured exclusively by chemical synthesis, and two main processes
are practiced today. The direct amination of chloroacetic acid
with a large excess of ammonia gives good yields of glycine without producing
large amounts of di- and trialkylated products. This process is widely used in
China, where the main application of the glycine is as a raw material for the
herbicide glyphosate.
The other main process is the Strecker synthesis. The direct Strecker reaction of
formaldehyde and ammonium cyanide produces methylene amino acetonitrile,
which must be hydrolyzed in two stages to produce glycine . A more efficient
approach is to aminate the intermediate glycolonitrile, followed by hydrolysis].
An alternative method, which is more often applied for the homologous amino
acids, is the Bucherer–Bergs reaction. Reaction of formaldehyde and ammonium
carbonate or bicarbonate gives the intermediate hydantoin, which can be hydrolyzed
to glycine in a separate step. |
|
Biological Functions
|
The principal function of glycine is as a precursor to proteins. It is also a building block to numerous natural products.As a biosynthetic intermediate In higher eukaryotes, D-Aminolevulinic acid, the key precursor to porphyrins, is biosynthesized from glycine and succinyl-CoA. Glycine provides the central C2N subunit of all purines. As a neurotransmitter Glycine is an inhibitory neurotransmitter in the central nervous system, especially in the spinal cord, brainstem, and retina. When glycine receptors are activated, chloride enters the neuron via ionotropic receptors, causing an Inhibitory postsynaptic potentia (IPSP). Strychnine is a strong antagonist at ionotropic glycine receptors, whereas bicuculline is a weak one. Glycine is a required coagonist along with glutamate for NMDA receptors. In contrast to the inhibitory role of glycine in the spinal cord, this behaviour is facilitated at the (NMDA) glutaminergic receptors which are excitatory. The LD50 of glycine is 7930 mg / kg in rats (oral), and it usually causes death by hyperexcitability. . |
|
General Description
|
White crystals. |
|
Air & Water Reactions
|
Water soluble. |
|
Reactivity Profile
|
An amino acid. A 0.2M aqueous solution has a pH of 4.0., so acts as a weak acid. Has characteristics of both acid and base. |
|
Hazard
|
Use in fats restricted to 0.01%. |
|
Fire Hazard
|
LOW. Ignites at very high temperatures. |
|
Pharmaceutical Applications
|
Glycine is routinely used as a cofreeze-dried excipient in protein
formulations owing to its ability to form a strong, porous, and
elegant cake structure in the final lyophilized product. It is one
of the most frequently utilized excipients in freeze-dried injectable
formulations owing to its advantageous freeze-drying properties.
Glycine has been investigated as a disintegration accelerant in
fast-disintegrating formulations owing to its excellent wetting
nature.It is also used as a buffering agent and conditioner in
cosmetics.
Glycine may be used along with antacids in the treatment of
gastric hyperacidity, and it may also be included in aspirin
preparations to aid the reduction of gastric irritation. |
|
Biological Activity
|
One of the major inhibitory neurotransmitters in the mammalian CNS, predominantly active in the spinal cord and brain stem. Also acts as a modulator of excitatory amino acid transmission mediated by NMDA receptors. Also available as part of the NMDA Receptor - Glycine Site Tocriset? . |
|
Biochem/physiol Actions
|
Glycine has a pivotal role in lowering the plasma lipid levels in diabetic and obese patients by activating the CNS. During brain hypoxia glycine can stabilize the energetics disturbances in brain mitochondria. It also increases the in vitro development of porcine blastocysts when used along with glucose. |
|
Safety Profile
|
Moderately toxic by
intravenous route. Mildly toxic by ingestion.
Mutation data reported. When heated to
decomposition it emits toxic fumes of NOx. |
|
Safety
|
Glycine is used as a sweetener, buffering agent, and dietary
supplement. The pure form of glycine is moderately toxic by the
IV route and mildly toxic by ingestion.
Systemic absorption of glycine irrigation solutions can lead to
disturbances of fluid and electrolyte balance and cardiovascular and
pulmonary disorders.
LD50 (mouse, IP): 4.45 g/kg
LD50 (mouse, IV): 2.37 g/kg
LD50 (mouse, oral): 4.92 g/kg
LD50 (mouse, SC): 5.06 g/kg
LD50 (rat, IV): 2.6 g/kg
LD50 (rat, oral): 7.93 g/kg
LD50 (rat, SC): 5.2 g/kg |
|
storage
|
Glycine starts to decompose at 233°C. Store in well-closed
containers. Glycine irrigation solutions (95–105% glycine) should
be stored in single dose containers, preferably type I or type II glass. |
|
Purification Methods
|
Crystallise glycine from distilled water by dissolving at 90-95o, filtering, cooling to about -5o, and draining the crystals centrifugally. Alternatively, crystallise it from distilled water by addition of MeOH or EtOH (e.g. 50g dissolved in 100mL of warm water, and 400mL of MeOH is added). The crystals are washed with MeOH or EtOH, then with diethyl ether. Likely impurities are ammonium glycinate, iminodiacetic acid, nitrilotriacetic acid or/and ammonium chloride. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 1955 1961, Beilstein 4 IV 2349.] |
|
Degradation
|
Glycine is degraded via three pathways. The predominant pathway in animals and plants involves the glycine cleavage enzyme Glycine + tetra hydro folate + NAD+ → CO2 + NH4+ + N5,N10-Methylene tetra hydrofolate + NADH + H+ In the second pathway, glycine is degraded in two steps. The first step is the reverse of glycine biosynthesis from serine with serine hydroxymethyl transferase. Serine is then converted to pyruvate by serine dehydratase. In the third pathway of glycine degradation, glycine is converted to glyoxylate by D-amino acid oxidase. Glyoxylate is then oxidized by hepatic lactate dehydrogenase to oxalate in an NAD+-dependent reaction. The half-life of glycine and its elimination from the body varies significantly based on dose. In one study, the half-life was between 0.5 and 4.0 hours. |
|
Presence in space
|
The detection of glycine in the interstellar medium has been debated . In 2008, the glycine - like molecule amino aceto nitrile was discovered in the Large Molecule Heimat, a giant gas cloud near the galactic center in the constellation Sagittarius by the Max Planck Institute for Radio Astronomy . In 2009, glycine sampled in 2004 from comet Wild 2 by the NASA spacecraft Stardust was confirmed, the first discovery of extraterrestrial glycine. That mission's results bolstered the theory of panspermia, which claims that the "seeds" of life are widespread throughout the universe. |
|
Incompatibilities
|
Glycine may undergo Maillard reactions with amino acids to
produce yellowing or browning. Reducing sugars will also interact
with secondary amines to form an imine, but without any
accompanying yellow-brown discoloration. |
|
Regulatory Status
|
GRAS listed. Accepted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (IM, IV, SC
injections; oral; rectal) and approved for irrigant solutions. Included
in parenteral (powders for injection; solutions for injection;
vaccines; kits for implant) and nonparenteral (orodispersible
tablets/oral lyophilizate; powders for inhalation; powders for oral
solution; tablets) formulations licensed in the UK. |