A series of 1D, 2D and 3D coordination polymers based on a 5-(benzonic-4-ylmethoxy)isophthalic acid: syntheses, structures and photoluminescence
Ying-Ying Liu , Jing Li , Jian-Fang Ma *, Ji-Cheng Ma and Jin Yang *
, CrystEngComm, 2012, 14, 169-177
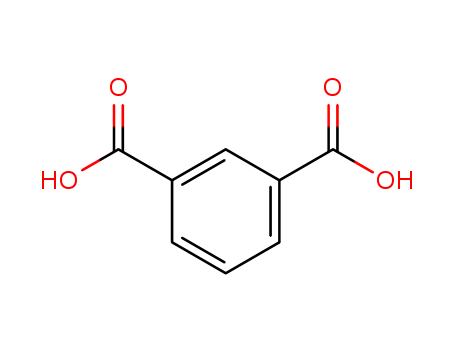
Seven new coordination polymers, namely, [Zn(HL)(H2O)] (1), [Zn(HL)(phen)]·1.5H2O (2), [Zn(HL)(L1)] (3), [Zn2(HL)2(L2)2]·2H2O (4), [Zn(HL)(L3)0.5] (5), [Zn(HL)(L4)] (6) and [Cu2(L)(OH)(H2O)]·0.5H2O (7) (H3L = 5-(benzonic-4-ylmethoxy) isophthalic acid, phen = 1,10-phenathroline, L1 = 1,2-bis(1,2,4-triazole-1-yl)ethane, L2 = 1,3-bis(1,2,4-triazole-1-yl)propane, L3 = 1,6-bis(1,2,4-triazole-1-yl)hexane and L4 = 4,4′-bis(1,2,4-triazole-1-ylmethyl)biphenyl), have been hydrothermally synthesized and characterized by single-crystal X-ray diffraction.
Fabrication of three dimensional (3D) hierarchical Ag/WO3 flower-like catalyst materials for the selective oxidation of m-xylene to isophthalic acid
Acharyya, Shankha S.,Ghosh, Shilpi,Bal, Rajaram
, p. 5998 - 6001 (2015)
A three dimensional (3D) hierarchical si...
Substituent effect on the assembly of coordination polymers containing isophthalic acid and its derivatives
Dong-Sheng Zhou,a Fang-Kuo Wang,ab Shi-Yao Yang,*a Zhao-Xiong Xiea and Rong-Bin Huanga
, CrystEngComm, Issue 11, 2009
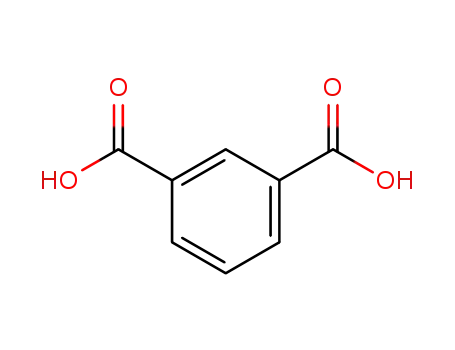
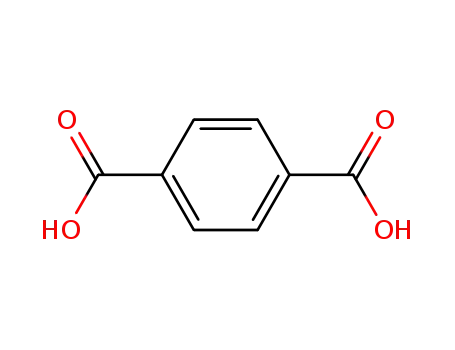
Four coordination polymers [Zn4(H2O)(ip)4(py)6]n1, {[Zn2(hip)2(py)4]2·(py)}n2, [Zn(tbip)(py)2]n3 and [Mn(tbip)(py)2]n4 (H2ip = isophthalic acid, H2hip = 5-hydroxyisophthalic acid, H2tbip = 5-tert-butylisophthalic acid, py = pyridine), have been hydrothermally synthesized and characterized. The substituents on isophthalic acid influence the coordination environments of metal ions and the coordination modes of the carboxyls, and thus determine the structures of the coordination polymers.