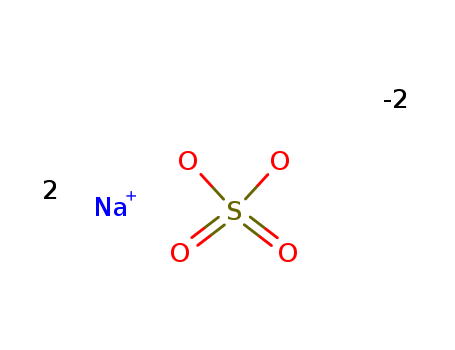
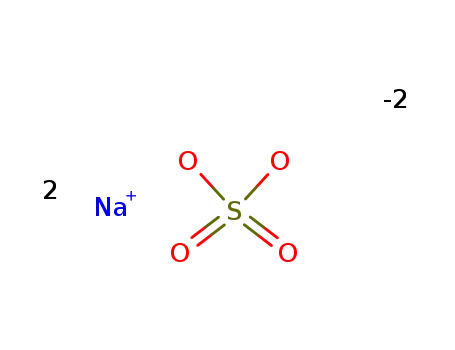
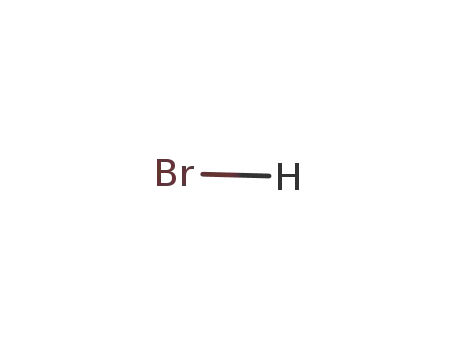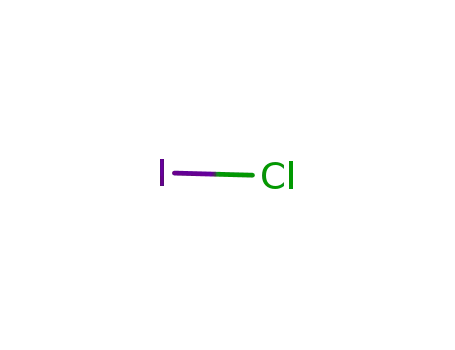Quality manufacturer supply Sodium sulfate 7757-82-6 in stock with high standard
- Molecular Formula:Na2SO4
- Molecular Weight:142.043
- Appearance/Colour:white crystals or powder
- Vapor Pressure:3.35E-05mmHg at 25°C
- Melting Point:884 °C
- Refractive Index:1.484
- Boiling Point:330 °C at 760 mmHg
- PSA:88.64000
- Density:2.68 g/mL at 25 °C(lit.)
- LogP:-0.25720
Sodium sulfate(Cas 7757-82-6) Usage
| Description |
Sodium sulfate (Na₂SO₄), also known as disodium sulfate or sulfate of soda, is a white crystalline solid that is highly soluble in water. It is a versatile inorganic compound used extensively across various industries. |
| Production Methods |
Sodium sulfate can be derived from two main sources:
Natural Sources: Extracted from naturally occurring brines or crystalline evaporite deposits.
Chemical Byproduct: Produced as a byproduct of chemical processes such as acid recycling in the battery industry.
|
| Uses |
Detergents and Soaps: Used as a filler in powdered laundry detergents due to its high solubility and low cost.
Glass Manufacturing: Functions as a fining agent to remove small air bubbles from molten glass.
Textile Industry: Plays a role in dyeing textiles, helping to even out dye penetration.
Alkali Activation Processes: Sodium sulfate is an eco-friendly activator for alkali-activated composites (AACs) and geopolymers. It plays a key role in binding properties of alternative binders to Ordinary Portland Cement (OPC).
|
| Hazards and Safety |
Toxicity: Sodium sulfate is generally non-toxic but can cause eye and skin irritation upon contact. It is considered safe for general industrial and pharmaceutical uses but should still be handled carefully to avoid accidental exposure.
Environmental Considerations: While not highly hazardous, sodium sulfate is considered an environmental contaminant if released in large quantities due to its high solubility and potential effects on water ecosystems. |
| Physiological Function as a Laxative |
Sodium sulfate works as an osmotic laxative by increasing the retention of water in the intestines, softening stool, and promoting bowel movement. This effect makes it valuable in bowel preparations before medical procedures like colonoscopies. |
InChI:InChI=1/2Na.H2O4S/c;;1-5(2,3)4/h;;(H2,1,2,3,4)/q2*+1;/p-2
Jilin Tianyun New Materials Co., Ltd. is a professional production enterprise that production, processing, and export, mainly engaged in various organic chemicals. The company has a wealth of professional experience, reliable product quality, reasonable prices, and products are exported to countries around the world, which can meet your diverse needs. In our business cooperation with clients, we always adhere to the principle of equality and mutual benefit, and are deeply trusted and recognized by our clients. If you are interested in our company and our products, please feel free to visit our website or contact us directly for more information.We look forward to establishing a long-term stable and win-win business relationship with you.
7757-82-6 Relevant articles
How does sodium sulfate crystallize? Implications for the decay and testing of building materials
Carlos Rodriguez-Navarro a , Eric Doehne a , Eduardo Sebastian b
, Cement and Concrete Research Volume 30, Issue 10, October 2000, Pages 1527-1534
Data from the environmental scanning electron microscope (ESEM) show no hydration phenomena following wetting of thenardite; instead, thenardite dissolution occurs, followed by thenardite plus mirabilite crystallization upon drying. These results offer new insight into how damage is caused by sodium sulfate in natural geological, archaeological, construction and engineering contexts. They also help explain some of the controversial results of various commonly used sodium sulfate crystallization tests.
Physico-mechanical and microstructural properties of sodium sulfate activated materials: A review
Adeyemi Adesina a , Cyriaque Rodrigue Kaze b
, Construction and Building Materials Volume 295, 9 August 2021, 123668
For example, the use of SS has been found to yield lower workability and higher shrinkage thereby making AACs made with such activator(s) suitable for specific applications where higher workability and lower shrinkage are required. On the other hand, the evolution of AACs over the years has shown that alternative sustainable and cheaper alkali activators can be used as a replacement for the conventional ones. Some of the alternative activators for AAC are sodium carbonate (SC), lime (LM), and sodium sulfate (SST). The low alkalinity of these alternative activators compared to the conventional ones makes their utilization on a large scale more practical and safer.
7757-82-6 Process route
-
- 10035-10-6,12258-64-9
hydrogen bromide
Conditions
| Conditions |
Yield |
|
With water; exclusion of oxygen, excess of thiosulfate, too;
|
|
|
With H2O; exclusion of oxygen, excess of thiosulfate, too;
|
|
-
- 7790-99-0
Iodine monochloride
-
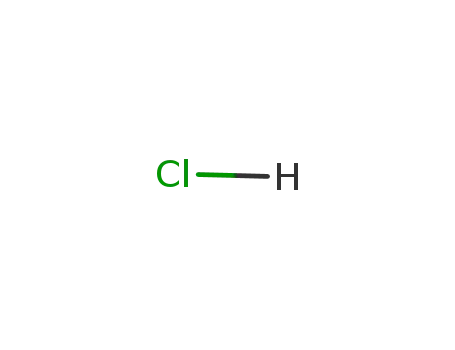
- 7647-01-0,15364-23-5
hydrogenchloride
-
- 10034-85-2
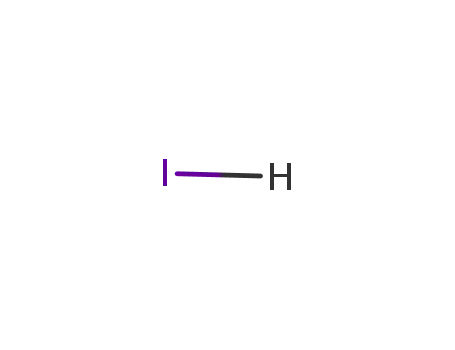
hydrogen iodide
7757-82-6 Upstream products
7757-82-6 Downstream products
-
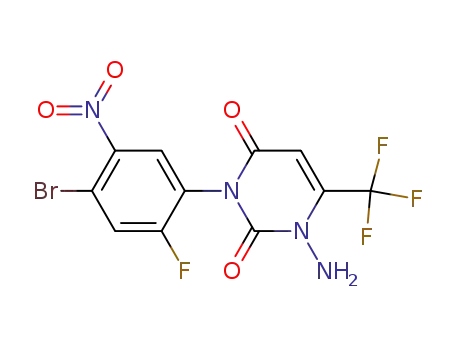
353240-66-1
1-amino-3-(4-bromo-2-fluoro-5-nitrophenyl)-6-tri-fluoromethyl-2,4-(1H,3H)-pyrimidinedione
-
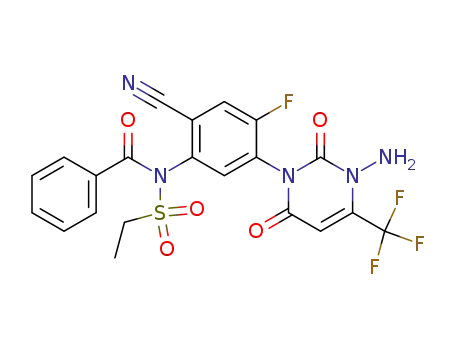
364592-74-5
N-[5-(3-amino-2,6-dioxo-4-trifluoromethyl-3,6-dihydro-1(2H)-pyrimidinyl)-2-cyano-4-fluoro-phenyl]-N-benzoyl-1-ethanesulphonamide
-
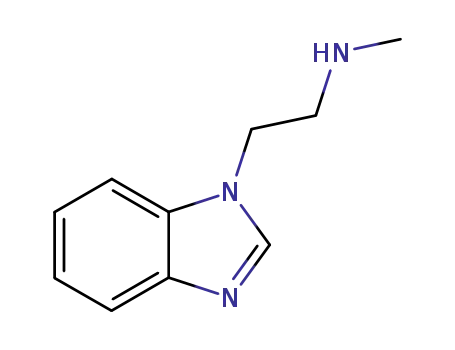
99168-05-5
1-(2-methylamino-ethyl)-1H-benzo[d]imidazole
-
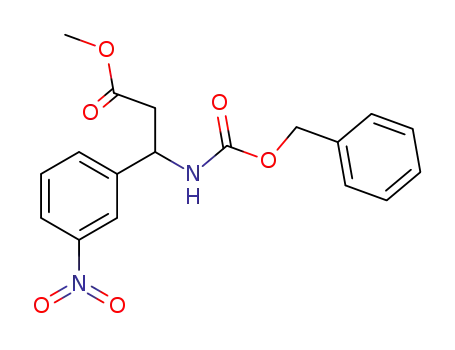
259195-70-5
methyl 3-benzyloxycarbonylamino-3-(3-nitrophenyl)propionate