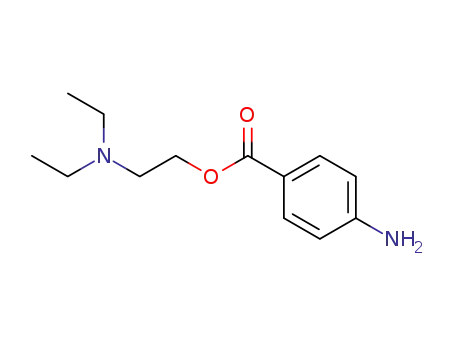
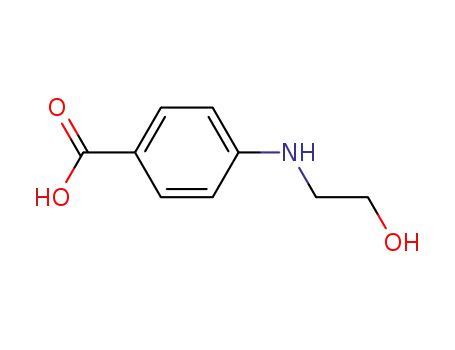
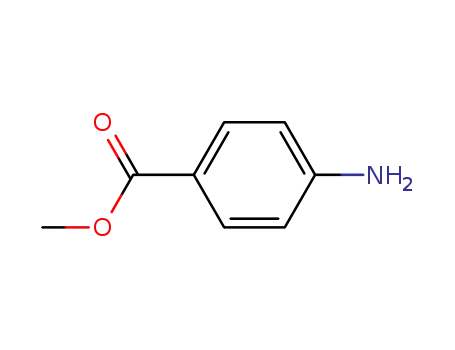
Synthesis and antimycobacterial activity of some coupling products from 4-aminobenzoic acid hydrazones
Ş.Güniz Küçükgüzel a , Sevim Rollas a , Ilkay Küçükgüzel a , Muammer Kiraz b
, European Journal of Medicinal Chemistry Volume 34, Issue 12 , December 1999, Pages 1093-1100
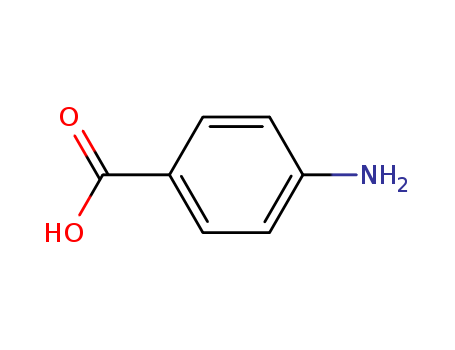
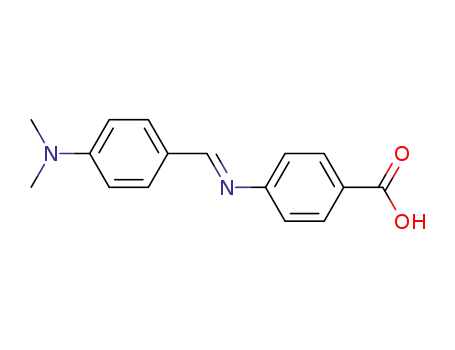
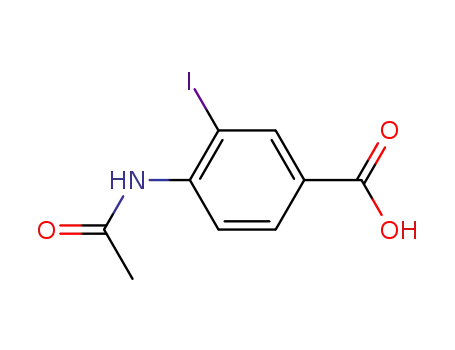
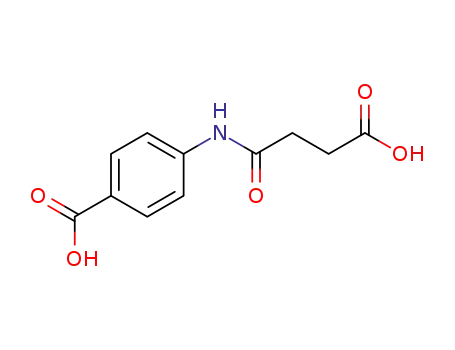
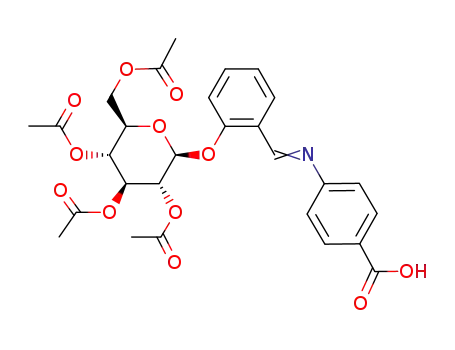
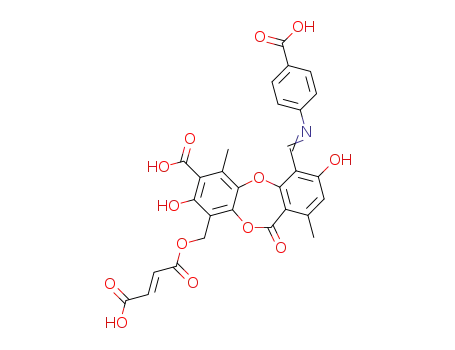
Antituberculosis and antibacterial effects have been shown with various 4-aminobenzoic acid substituted benzalhydrazones [1]. Furthermore, the coupling products starting from diazonium salts of 4-aminobenzoic acid hydrazide substituted benzalhydrazones with indole have also been reported to possess promising antibacterial and antitubercular activities [2]. These observations prompted us to synthesize some novel coupling products of 4-aminobenzoic acid hydrazide substituted benzalhydrazone derivatives.
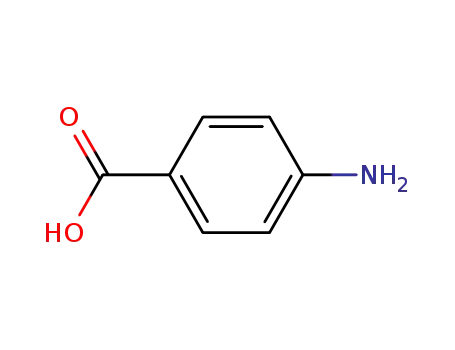
Synthesis and antibacterial activity of some Schiff bases derived from 4-aminobenzoic acid
J Parekh, P Inamdhar, R Nair, S Baluja
, Journal of the Serbian Chemical Society 2005 Volume 70, Issue 10, Pages: 1155-1162
The following Schiff bases have been synthesized: (1) 4-(2-chlorobenzylidene)amino benzoic acid JP1, (2) 4 (furan-2-ylmethylene)amino benzoic acid JP2, (3) 4-[(3-phenylallylidene)amino]benzoic acid JP3, (4) 4 (2-hydroxybenzylidene)amino benzoic acid JP4, (5) 4 (4-hydroxy-3-methoxybenzylidene)amino benzoic acid JP5 and (6) 4 (3-nitrobenzylidene)amino benzoic acid JP6.
Sulfonated covalent triazine polymer loaded with Pd nanoparticles as a bifunctional catalyst for one pot hydrogenation esterification reaction
Ravi, S.,Raza, A. Ahmed,Sheriff, A. K. Ibrahim,Tajudeen, S. Syed
, (2021)
Highly dispersed Pd nanoparticles over c...
Cytochrome P450 CYP199A4 from Rhodopseudomonas palustris Catalyzes Heteroatom Dealkylations, Sulfoxidation, and Amide and Cyclic Hemiacetal Formation
Coleman, Tom,Wong, Siew Hoon,Podgorski, Matthew N.,Bruning, John B.,De Voss, James J.,Bell, Stephen G.
, p. 5915 - 5927 (2018)
The cytochrome P450 enzymes execute a ra...
Ruthenium nanoparticles supported over mesoporous TiO2 as an efficient bifunctional nanocatalyst for esterification of biomass-derived levulinic acid and transfer-hydrogenation reactions
Mandi, Usha,Salam, Noor,Kundu, Sudipta K.,Bhaumik, Asim,Islam, Sk. Manirul
, p. 73440 - 73449 (2016)
We have supported ruthenium nanoparticle...
Kinetic and mechanistic investigations and thermodynamic quantities for different steps involved in the mechanism of oxidation of procainamide by hexacyanoferrate(III) in aqueous alkaline medium: a spectrophotometric study
Meti,Lamani,Naikar,Sutar,Nandibewoor,Chimatadar
, p. 1485 - 1493 (2015)
The kinetics of oxidation of procainamid...