Factory supply Sodium metabisulfite 7681-57-4 with sufficient stock and high standard
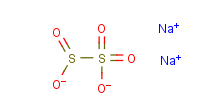
- Molecular Formula:Na2O5S2
- Molecular Weight:190.10
- Appearance/Colour:white crystalline powder with a sulfur smell
- Melting Point:>170 °C (begins at 150 °C)
- PSA:124.92000
- Density:1.48 g/cm3
- LogP:0.27220
Sodium metabisulfite(Cas 7681-57-4) Usage and Factory
|
Chemical Description
|
Sodium metabisulfite (Na₂S₂O₅) is an inorganic compound commonly used as a disinfectant, antioxidant, and preservative across several industries. It appears as a white powder with a faint rotten egg odor (due to sulfur dioxide release), and it forms sodium bisulfite when dissolved in water. |
| Manufacturing Method |
Sodium metabisulfite is produced by reacting sulfur dioxide with sodium carbonate (soda ash) or sodium hydroxide, followed by purification and crystallization. The reaction can be represented as: Na2CO3 + 2SO2 → Na2S2O5 + CO2. |
| Uses |
Food Industry: Used as a preservative (E223) in dried fruits, baked goods, jams, and potato products. It helps inhibit microbial growth and oxidation of food products.
Pharmaceutical Industry: Functions as an antioxidant to stabilize active ingredients in medications, including parenteral (injectable), oral, and topical formulations.
Disinfectant: Employed as a sterilizing agent in water treatment plants, especially for purifying wastewater. |
| Hazards and Safety |
Toxicity: Sodium metabisulfite poses inhalation risks, especially for individuals with asthma or sulfite allergies. It may irritate the respiratory tract and cause allergic reactions.
Carcinogenicity: Some studies suggest genotoxic effects, but it is not classified as a confirmed carcinogen.
Regulatory Status: It is Generally Recognized As Safe (GRAS) by the FDA for use as a food additive. It is also approved by the European Union and listed in various global databases, including the FDA's Inactive Ingredients Database. |
| Hazardous Substance Classification |
Sodium metabisulfite is included in the hazardous substance list due to its classification by organizations like ACGIH (American Conference of Governmental Industrial Hygienists), DOT (Department of Transportation), NIOSH (National Institute for Occupational Safety and Health), and IARC (International Agency for Research on Cancer). |
| Purification Methods |
Sodium metabisulfite is purified through crystallization techniques, including dissolving in water and recovering crystals through evaporation or precipitation with alcohol solutions. |
InChI:InChI=1/2Na.H2O5S2/c;;1-6(2)7(3,4)5/h;;(H,1,2)(H,3,4,5)/q2*+1;/p-2
Jilin Tianyun New Materials Co., Ltd. is a professional production enterprise that production, processing, and export, mainly engaged in various organic chemicals. The company has a wealth of professional experience, reliable product quality, reasonable prices, and products are exported to countries around the world, which can meet your diverse needs. In our business cooperation with clients, we always adhere to the principle of equality and mutual benefit, and are deeply trusted and recognized by our clients. If you are interested in our company and our products, please feel free to visit our website or contact us directly for more information.We look forward to establishing a long-term stable and win-win business relationship with you.
7681-57-4 Relevant articles
Recent advances in sulfonylation reactions using potassium/sodium metabisulfite
Shengqing Ye a, Min Yang ORCID logob and Jie Wu
, Chem. Commun., 2020, 56, 4145-4155
Recently, sulfonylation reactions using potassium/sodium metabisulfite as the sulfur dioxide surrogate have been developed rapidly. In most cases, the transformations go through radical processes with the insertion of sulfur dioxide under mild conditions. Additionally, transition metal catalysis is applied in the reactions for the synthesis of sulfonyl-containing compounds. Among the approaches, photoinduced conversions under visible light or ultraviolet irradiation are also involved. In this updated report, the insertion of sulfur dioxide from potassium metabisulfite or sodium metabisulfite is summarized.
Allergic contact dermatitis caused by sodium metabisulfite: a challenging allergen. A case series and literature review
Juan García-Gavín, Joana Parente, An Goossens
, Contact dermatitis, Volume67, Issue5 November 2012 Pages 260-269
One hundred and twenty-four (4.5%) of 2763 patients patch tested positively to sodium metabisulfite. The most frequent localizations of the lesions were the face (40.3%) and the hands (24.2%). Six patients also reported systemic symptoms. Thirteen cases (10.5%) were occupational, 10 of them presenting with hand eczema. Sodium metabisulfite was the single allergen found in 76 cases (61.3%). The reactions were considered to be relevant in 80 cases (64.5%), of which 11 were occupational.